Vehicle-dependent disposition kinetics of fluoranthene in Fisher-344 rats
- PMID: 18441404
- PMCID: PMC2760079
- DOI: 10.3390/ijerph5010041
Vehicle-dependent disposition kinetics of fluoranthene in Fisher-344 rats
Abstract
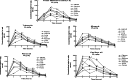
The objective of this study was to evaluate how the vehicles of choice affect the pharmacokinetics of orally administered Fluoranthene [FLA] in rats. Fluoranthene is a member of the family of Polycyclic Aromatic Hydrocarbon chemicals. Fluoranthene exposure to humans may occur as a result of cigarette smoking, consumption of contaminated food and water, heating woods in stoves and boilers, industrial sources such as coal gasification, carbon and graphite electrode manufacturing. Adult male Fisher-344 rats were given single oral doses of 25 and 50 microg/kg FLA in tricaprylin, peanut oil, cod liver oil, Tween 80/isotonic saline (1:5) and 2% Alkamuls-EL620 through gavage. After administration, the rats were housed individually in metabolic cages and sacrificed at 2, 4, 6, 8, 10 and 12 hours post FLA exposure. Blood, lung, liver, small intestine, adipose tissue samples, urine, and feces were collected at each time point. Samples were subjected to a liquid-liquid extraction using methanol, chloroform, and water. The extracts were analyzed by a reverse-phase HPLC, equipped with a fluorescence detector. The results revealed a dose-dependent increase in FLA concentrations in plasma and tissues for all the vehicles used. Plasma and tissue FLA concentrations were greater for peanut oil; cod liver oil, and tricaprylin vehicles compared to Alkamuls (p < 0.05), and Tween 80/isotonic saline (1:5). Most of the FLA administered through peanut oil, cod liver oil and tricaprylin was cleared from the body by 8 hours (90%) and 12 hours (80%) post administration for the 25 microg/kg and 50 microg/kg dose groups, respectively. With both doses employed, the metabolism of FLA was highest when cod liver oil was used as a vehicle and lowest in vehicles containing detergent/water [cod liver oil > peanut oil > tricaprylin > alkamuls > Tween 80/isotonic saline (1:5)]. These findings suggest that uptake and elimination of FLA is accelerated when administered through oil-based vehicles. The low uptake of FLA from Alkamuls and Tween 80/isotonic saline may have been a result of the poor solubility of the chemical. In summary, our findings reiterate that absorption characteristics of FLA were governed by the dose as well as the dosing vehicle. The vehicle-dependent bioavailability of FLA suggests a need for the judicious selection of vehicles in evaluating oral toxicity studies for risk assessment purposes.
Figures



Similar articles
-
Effect of dietary fat on metabolism and DNA adduct formation after acute oral exposure of F-344 rats to fluoranthene.J Nutr Biochem. 2007 Apr;18(4):236-49. doi: 10.1016/j.jnutbio.2006.04.001. Epub 2006 Jun 16. J Nutr Biochem. 2007. PMID: 16781860
-
Fluoranthene-induced neurobehavioral toxicity in F-344 rats.Int J Toxicol. 2003 Jul-Aug;22(4):263-76. doi: 10.1080/10915810305114. Int J Toxicol. 2003. PMID: 12933321
-
NTP Comparative Toxicology Studies of Corn Oil, Safflower Oil, and Tricaprylin (CAS Nos. 8001-30-7, 8001-23-8, and 538-23-8) in Male F344/N Rats as Vehicles for Gavage.Natl Toxicol Program Tech Rep Ser. 1994 Apr;426:1-314. Natl Toxicol Program Tech Rep Ser. 1994. PMID: 12616305
-
Final report on the safety assessment of trilaurin, triarachidin, tribehenin, tricaprin, tricaprylin, trierucin, triheptanoin, triheptylundecanoin, triisononanoin, triisopalmitin, triisostearin, trilinolein, trimyristin, trioctanoin, triolein, tripalmitin, tripalmitolein, triricinolein, tristearin, triundecanoin, glyceryl triacetyl hydroxystearate, glyceryl triacetyl ricinoleate, and glyceryl stearate diacetate.Int J Toxicol. 2001;20 Suppl 4:61-94. Int J Toxicol. 2001. PMID: 11800053 Review.
-
Final report on the safety assessment of Peanut (Arachis hypogaea) Oil, Hydrogenated Peanut Oil, Peanut Acid, Peanut Glycerides, and Peanut (Arachis hypogaea) Flour.Int J Toxicol. 2001;20 Suppl 2:65-77. doi: 10.1080/10915810160233776. Int J Toxicol. 2001. PMID: 11558642 Review.
Cited by
-
Olive oil prevents benzo(a)pyrene [B(a)P]-induced colon carcinogenesis through altered B(a)P metabolism and decreased oxidative damage in Apc(Min) mouse model.J Nutr Biochem. 2016 Feb;28:37-50. doi: 10.1016/j.jnutbio.2015.09.023. Epub 2015 Oct 22. J Nutr Biochem. 2016. PMID: 26878781 Free PMC article.
-
Prenatal exposure to polycyclic aromatic hydrocarbons and executive functions at school age: Results from a combined cohort study.Int J Hyg Environ Health. 2024 Jul;260:114407. doi: 10.1016/j.ijheh.2024.114407. Epub 2024 Jun 15. Int J Hyg Environ Health. 2024. PMID: 38879913 Free PMC article.
-
Dietary fat-influenced development of colon neoplasia in Apc Min mice exposed to benzo(a)pyrene.Toxicol Pathol. 2009 Dec;37(7):938-46. doi: 10.1177/0192623309351722. Toxicol Pathol. 2009. PMID: 19841130 Free PMC article.
-
Human in Vivo Pharmacokinetics of [(14)C]Dibenzo[def,p]chrysene by Accelerator Mass Spectrometry Following Oral Microdosing.Chem Res Toxicol. 2015 Jan 20;28(1):126-34. doi: 10.1021/tx5003996. Epub 2014 Dec 10. Chem Res Toxicol. 2015. PMID: 25418912 Free PMC article. Clinical Trial.
References
-
- Dolusio JT, Tan GH, Billups NF, Diamond L. Drug absorption. II. Effect of fasting on intestinal drug absorption. J. Pharmacol. Sci. 1969;58:1200–1202. - PubMed
-
- Balimane PV, Chong S, Morrison RA. Current methodologies used for evaluation of intestinal permeability and absorption. Journal of Pharmacol. and Toxicol. Methods. 2000;44:301–312. - PubMed
-
- Gad SC, Chengelis CP. Acute toxicology of testing perspectives and horizons. Caldwell, N. J: Telford Press; 1988.
-
- O’Hara TM, Borzelleca JF, Clarke EC, Sheppard MA, Condie LWA. CCl4/CHCl3 interaction study in isolated hepatocytes: selection of a vehicle. Fundam. Appl. Toxicol. 1989;13:605–615. - PubMed
-
- Braak K, Frey H-H. Effects of solvents and detergents on the contractions of isolated smooth muscle preparations. J. Pharm. Pharmacol. 1990;42:837–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- D34HP00003/PHS HHS/United States
- G12 RR003032/RR/NCRR NIH HHS/United States
- S11 ES014156/ES/NIEHS NIH HHS/United States
- U54 MD007593/MD/NIMHD NIH HHS/United States
- 1R03CA130112-01/CA/NCI NIH HHS/United States
- 5T32HL007735-12/HL/NHLBI NIH HHS/United States
- T32 HL007735/HL/NHLBI NIH HHS/United States
- S11ES014156-01/ES/NIEHS NIH HHS/United States
- R03 CA130112/CA/NCI NIH HHS/United States
- 1R15ES012168/ES/NIEHS NIH HHS/United States
- R15 ES012168/ES/NIEHS NIH HHS/United States
- G12RRO3032/PHS HHS/United States
- R01 CA142845/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
