Transcription analysis in the MeLiM swine model identifies RACK1 as a potential marker of malignancy for human melanocytic proliferation
- PMID: 18442364
- PMCID: PMC2387171
- DOI: 10.1186/1476-4598-7-34
Transcription analysis in the MeLiM swine model identifies RACK1 as a potential marker of malignancy for human melanocytic proliferation
Abstract
Background: Metastatic melanoma is a severe disease. Few experimental animal models of metastatic melanoma exist. MeLiM minipigs exhibit spontaneous melanoma. Cutaneous and metastatic lesions are histologically similar to human's. However, most of them eventually spontaneously regress. Our purpose was to investigate whether the MeLiM model could reveal markers of malignancy in human melanocytic proliferations.
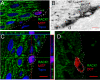
Results: We compared the serial analysis of gene expression (SAGE) between normal pig skin melanocytes and melanoma cells from an early pulmonary metastasis of MeLiM minipigs. Tag identification revealed 55 regulated genes, including GNB2L1 which was found upregulated in the melanoma library. In situ hybridisation confirmed GNB2L1 overexpression in MeLiM melanocytic lesions. GNB2L1 encodes the adaptor protein RACK1, recently shown to influence melanoma cell lines tumorigenicity. We studied the expression of RACK1 by immunofluorescence and confocal microscopy in tissues specimens of normal skin, in cutaneous and metastatic melanoma developped in MeLiM minipigs and in human patients. In pig and human samples, the results were similar. RACK1 protein was not detected in normal epidermal melanocytes. By contrast, RACK1 signal was highly increased in the cytoplasm of all melanocytic cells of superficial spreading melanoma, recurrent dermal lesions and metastatic melanoma. RACK1 partially colocalised with activated PKCalphabeta. In pig metastases, additional nuclear RACK1 did not associate to BDNF expression. In human nevi, the RACK1 signal was low.
Conclusion: RACK1 overexpression detected in situ in human melanoma specimens characterized cutaneous and metastatic melanoma raising the possibility that RACK1 can be a potential marker of malignancy in human melanoma. The MeLiM strain provides a relevant model for exploring mechanisms of melanocytic malignant transformation in humans. This study may contribute to a better understanding of melanoma pathophysiology and to progress in diagnosis.
Figures






Similar articles
-
RACK1, a clue to the diagnosis of cutaneous melanomas in horses.BMC Vet Res. 2012 Jun 29;8:95. doi: 10.1186/1746-6148-8-95. BMC Vet Res. 2012. PMID: 22747534 Free PMC article.
-
Canine melanoma diagnosis: RACK1 as a potential biological marker.Vet Pathol. 2013 Nov;50(6):1083-90. doi: 10.1177/0300985813490754. Epub 2013 Jun 4. Vet Pathol. 2013. PMID: 23735618
-
Functional mapping of the promoter region of the GNB2L1 human gene coding for RACK1 scaffold protein.Gene. 2009 Feb 1;430(1-2):17-29. doi: 10.1016/j.gene.2008.10.005. Epub 2008 Oct 21. Gene. 2009. PMID: 19000751
-
Melanoma-Bearing Libechov Minipig (MeLiM): The Unique Swine Model of Hereditary Metastatic Melanoma.Genes (Basel). 2019 Nov 9;10(11):915. doi: 10.3390/genes10110915. Genes (Basel). 2019. PMID: 31717496 Free PMC article. Review.
-
Role of In Vivo Reflectance Confocal Microscopy in the Analysis of Melanocytic Lesions.Acta Dermatovenerol Croat. 2018 Apr;26(1):64-67. Acta Dermatovenerol Croat. 2018. PMID: 29782304 Review.
Cited by
-
The Apoptotic, Angiogenic and Cell Proliferation Genes CD63, S100A6 e GNB2L1 are Altered in Patients with Endometriosis.Rev Bras Ginecol Obstet. 2018 Oct;40(10):606-613. doi: 10.1055/s-0038-1673364. Epub 2018 Oct 23. Rev Bras Ginecol Obstet. 2018. PMID: 30352458 Free PMC article.
-
RACK1 facilitates breast cancer progression by competitively inhibiting the binding of β-catenin to PSMD2 and enhancing the stability of β-catenin.Cell Death Dis. 2023 Oct 17;14(10):685. doi: 10.1038/s41419-023-06191-3. Cell Death Dis. 2023. PMID: 37848434 Free PMC article.
-
RACK1 depletion in a mouse model causes lethality, pigmentation deficits and reduction in protein synthesis efficiency.Cell Mol Life Sci. 2013 Apr;70(8):1439-50. doi: 10.1007/s00018-012-1215-y. Epub 2012 Dec 2. Cell Mol Life Sci. 2013. PMID: 23212600 Free PMC article.
-
Downregulation of receptor for activated C-kinase 1 (RACK1) suppresses tumor growth by inhibiting tumor cell proliferation and tumor-associated angiogenesis.Cancer Sci. 2011 Nov;102(11):2007-13. doi: 10.1111/j.1349-7006.2011.02065.x. Epub 2011 Sep 22. Cancer Sci. 2011. PMID: 21848913 Free PMC article.
-
Malignant features of minipig melanomas prior to spontaneous regression.Sci Rep. 2024 Apr 22;14(1):9240. doi: 10.1038/s41598-024-59741-w. Sci Rep. 2024. PMID: 38649394 Free PMC article.
References
-
- Vincent-Naulleau S, Le Chalony C, Leplat JJ, Bouet S, Bailly C, Spatz A, Vielh P, Avril MF, Tricaud Y, Gruand J, et al. Clinical and histopathological characterization of cutaneous melanomas in the melanoblastoma-bearing Libechov minipig model. Pigment Cell Res. 2004;17:24–35. doi: 10.1046/j.1600-0749.2003.00101.x. - DOI - PubMed
-
- Geffrotin C, Crechet F, Le Roy P, Le Chalony C, Leplat JJ, Iannuccelli N, Barbosa A, Renard C, Gruand J, Milan D, et al. Identification of five chromosomal regions involved in predisposition to melanoma by genome-wide scan in the MeLiM swine model. Int J Cancer. 2004;110:39–50. doi: 10.1002/ijc.20053. - DOI - PubMed
-
- Du ZQ, Vincent-Naulleau S, Gilbert H, Vignoles F, Crechet F, Shimogiri T, Yasue H, Leplat JJ, Bouet S, Gruand J, et al. Detection of novel quantitative trait loci for cutaneous melanoma by genome-wide scan in the MeLiM swine model. Int J Cancer. 2007;120:303–320. doi: 10.1002/ijc.22467. - DOI - PubMed
-
- Le Chalony C, Renard C, Vincent-Naulleau S, Crechet F, Leplat JJ, Tricaud Y, Horak V, Gruand J, Le Roy P, Frelat G, Geffrotin C. CDKN2A region polymorphism and genetic susceptibility to melanoma in the melim swine model of familial melanoma. Int J Cancer. 2003;103:631–635. doi: 10.1002/ijc.10871. - DOI - PubMed
-
- Horak V, Fortyn K, Hruban V, Klaudy J. Hereditary melanoblastoma in miniature pigs and its successful therapy by devitalization technique. Cell Mol Biol (Noisy-le-grand) 1999;45:1119–1129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

