Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress
- PMID: 18442969
- PMCID: PMC2427341
- DOI: 10.1074/jbc.M802797200
Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress
Abstract
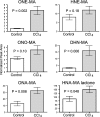
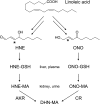
Oxidative stress-induced lipid peroxidation leads to the formation of cytotoxic and genotoxic 2-alkenals, such as 4-hydroxy-2-nonenal (HNE) and 4-oxo-2-nonenal (ONE). Lipid-derived reactive aldehydes are subject to phase-2 metabolism and are predominantly found as mercapturic acid (MA) conjugates in urine. This study shows evidence for the in vivo formation of ONE and its phase-1 metabolites, 4-oxo-2-nonen-1-ol (ONO) and 4-oxo-2-nonenoic acid (ONA). We have detected the MA conjugates of HNE, 1,4-dihydroxy-2-nonene (DHN), 4-hydroxy-2-nonenoic acid (HNA), the lactone of HNA, ONE, ONO, and ONA in rat urine by liquid chromatography-tandem mass spectrometry comparison with synthetic standards prepared in our laboratory. CCl(4) treatment of rats, a widely accepted animal model of acute oxidative stress, resulted in a significant increase in the urinary levels of DHN-MA, HNA-MA lactone, ONE-MA, and ONA-MA. Our data suggest that conjugates of HNE and ONE metabolites have value as markers of in vivo oxidative stress and lipid peroxidation.
Figures






Similar articles
-
Quantitation of mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites in a smoking cessation study.Free Radic Biol Med. 2010 Jan 1;48(1):65-72. doi: 10.1016/j.freeradbiomed.2009.10.025. Epub 2009 Oct 9. Free Radic Biol Med. 2010. PMID: 19819328 Free PMC article.
-
Glutathione conjugates of 4-hydroxy-2(E)-nonenal as biomarkers of hepatic oxidative stress-induced lipid peroxidation in rats.Free Radic Biol Med. 2005 Jun 1;38(11):1526-36. doi: 10.1016/j.freeradbiomed.2005.02.015. Free Radic Biol Med. 2005. PMID: 15890627
-
LC-MS/MS quantitation of mercapturic acid conjugates of lipid peroxidation products as markers of oxidative stress.Curr Protoc Toxicol. 2011 Feb 1;Chapter 17:Unit17.14.2. doi: 10.1002/0471140856.tx1714s45. Curr Protoc Toxicol. 2011. PMID: 21442005 Free PMC article.
-
The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis.Redox Biol. 2013 Jan 21;1(1):145-52. doi: 10.1016/j.redox.2013.01.007. Redox Biol. 2013. PMID: 24024147 Free PMC article. Review.
-
Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways.Mol Aspects Med. 2003 Aug-Oct;24(4-5):177-87. doi: 10.1016/s0098-2997(03)00012-8. Mol Aspects Med. 2003. PMID: 12892995 Review.
Cited by
-
Quantitation of mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites in a smoking cessation study.Free Radic Biol Med. 2010 Jan 1;48(1):65-72. doi: 10.1016/j.freeradbiomed.2009.10.025. Epub 2009 Oct 9. Free Radic Biol Med. 2010. PMID: 19819328 Free PMC article.
-
Vitamin C supplementation lowers urinary levels of 4-hydroperoxy-2-nonenal metabolites in humans.Free Radic Biol Med. 2011 Apr 1;50(7):848-53. doi: 10.1016/j.freeradbiomed.2011.01.004. Epub 2011 Jan 12. Free Radic Biol Med. 2011. PMID: 21236333 Free PMC article. Clinical Trial.
-
Association between ethylene oxide exposure and cognitive function in older adults from NHANES data.Sci Rep. 2025 Jan 28;15(1):3472. doi: 10.1038/s41598-025-87384-y. Sci Rep. 2025. PMID: 39875470 Free PMC article.
-
Mass spectrometry-based quantification of myocardial protein adducts with acrolein in an in vivo model of oxidative stress.Mol Nutr Food Res. 2011 Sep;55(9):1401-10. doi: 10.1002/mnfr.201100255. Epub 2011 Aug 2. Mol Nutr Food Res. 2011. PMID: 21809440 Free PMC article.
-
Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress.Br J Pharmacol. 2009 Oct;158(4):1062-73. doi: 10.1111/j.1476-5381.2009.00234.x. Epub 2009 May 5. Br J Pharmacol. 2009. PMID: 19422383 Free PMC article.
References
-
- Butterfield, D. A., and Sultana, R. (2007) J. Alzheimer's Dis. 12 61–72 - PubMed
-
- Picklo, M. J. S., and Montine, T. J. (2007) J. Alzheimer's Dis. 12 185–193 - PubMed
-
- Spiteller, G. (2007) Mol. Biotechnol. 37 5–12 - PubMed
-
- Benedetti, A., Comporti, M., and Esterbauer, H. (1980) Biochim. Biophys. Acta 620 281–296 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

