Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems
- PMID: 18442974
- PMCID: PMC2427356
- DOI: 10.1074/jbc.M802447200
Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems
Abstract
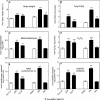
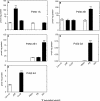
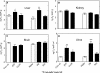
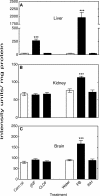
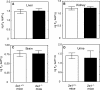
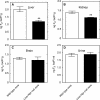
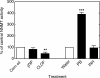
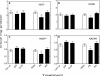
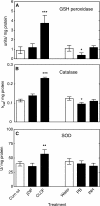
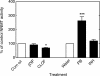
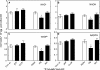
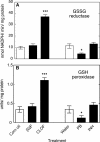
Reactive oxygen species (ROS) and oxidative stress have been considered in a variety of disease models, and cytochrome P450 (P450) enzymes have been suggested to be a source of ROS. Induction of P450s by phenobarbital (PB), beta-naphthoflavone (betaNF), or clofibrate in a mouse model increased ROS parameters in the isolated liver microsomes, but isoniazid treatment did not. However, when F(2)-isoprostanes (F(2)-IsoPs) were measured in tissues and urine, PB showed the strongest effect and betaNF had a measurable but weaker effect. The same trend was seen when an Nfr2-based transgene reporter sensitive to ROS was analyzed in the mice. This pattern had been seen earlier with F(2)-IsoPs both in vitro and in vivo with rats (Dostalek, M., Brooks, J. D., Hardy, K. D., Milne, G. L., Moore, M. M., Sharma, S., Morrow, J. D., and Guengerich, F. P. (2007) Mol. Pharmacol. 72, 1419-1424). One possibility for the general in vitro-in vivo discrepancy in oxidative stress found in both mice and rats is that PB treatment might attenuate protective systems. One potential candidate suggested by an mRNA microarray was nicotinamide N-methyltransferase. PB was found to elevate nicotinamide N-methyltransferase activity 3- to 4-fold in mice and rats and to attenuate levels of NAD(+), NADP(+), NADH, and NADPH in both species (20-40%), due to the enhanced excretion of (N-methyl)nicotinamide. PB also down-regulated glutathione peroxidase and glutathione reductase, which together constitute a key enzymatic system that uses NADPH in protecting against oxidative stress. These multiple effects on the protective systems are proposed to be more important than P450 induction in oxidative stress and emphasize the importance of studying in vivo models.
Figures
References
-
- Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., and Telser, J. (2007) Int. J. Biochem. Cell Biol. 39 44–84 - PubMed
-
- Palmer, G., and Reedijk, J. (1992) J. Biol. Chem. 267 665–677 - PubMed
-
- Jabs, T. (1999) Biochem. Pharmacol. 57 231–245 - PubMed
-
- Finkel, T., and Holbrook, N. J. (2000) Nature 408 239–247 - PubMed
-
- Lee, S. R., Yang, K. S., Kwon, J., Lee, C., Jeong, W., and Rhee, S. G. (2002) J. Biol. Chem. 277 20336–20342 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

