Regulation of intra-S phase checkpoint by ionizing radiation (IR)-dependent and IR-independent phosphorylation of SMC3
- PMID: 18442975
- PMCID: PMC2443663
- DOI: 10.1074/jbc.M802299200
Regulation of intra-S phase checkpoint by ionizing radiation (IR)-dependent and IR-independent phosphorylation of SMC3
Abstract
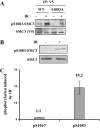
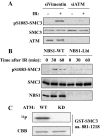
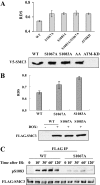
Structure maintenance of chromosome 1 (SMC1) is phosphorylated by ataxia telangiectasia-mutated (ATM) in response to ionizing radiation (IR) to activate intra-S phase checkpoint. A role of CK2 in DNA damage response has been implicated in many previous works, but the molecular mechanism for its activation is not clear. In the present work, we report that SMC3 is phosphorylated at Ser-1067 and Ser-1083 in vivo. Ser-1083 phosphorylation is IR-inducible, depends on ATM and Nijmegen breakage syndrome 1 (NBS1), and is required for intra-S phase checkpoint. Interestingly, Ser-1067 phosphorylation is constitutive and is not induced by IR but also affects intra-S phase checkpoint. Phosphorylation of Ser-1083 is weakened in cells expressing S1067A mutant, suggesting interplay between Ser-1067 and Ser-1083 phosphorylation in DNA damage response. Consistently, small interfering RNA knockdown of CK2 leads to attenuated phosphorylation of Ser-1067 as well as intra-S phase checkpoint defect. Our data provide evidence that phosphorylation of a core cohesin subunit SMC3 by ATM plays an important role in DNA damage response and suggest that a constitutive phosphorylation by CK2 may affect intra-S phase checkpoint by modulating SMC3 phosphorylation by ATM.
Figures


References
-
- Zhou, B. B., and Elledge, S. J. (2000) Nature 408 433–439 - PubMed
-
- Kastan, M. B., and Bartek, J. (2004) Nature 432 316–323 - PubMed
-
- Homma, M. K., and Homma, Y. (2005) Mol. Cell Biochem. 274 47–52 - PubMed
-
- Harvey, E. J., Li, N., and Ramji, D. P. (2007) Arterioscler. Thromb. Vasc. Biol. 27 806–812 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

