The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum
- PMID: 18442980
- PMCID: PMC2441541
- DOI: 10.1074/jbc.M800986200
The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum
Abstract
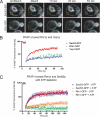
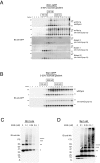
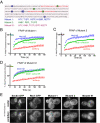
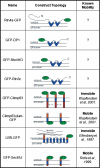
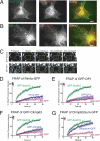
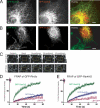
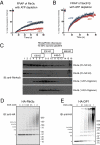
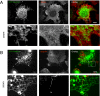
We recently identified a class of membrane proteins, the reticulons and DP1/Yop1p, which shape the tubular endoplasmic reticulum (ER) in yeast and mammalian cells. These proteins are highly enriched in the tubular portions of the ER and virtually excluded from other regions. To understand how they promote tubule formation, we characterized their behavior in cellular membranes and addressed how their localization in the ER is determined. Using fluorescence recovery after photobleaching, we found that yeast Rtn1p and Yop1p are less mobile in the membrane than normal ER proteins. Sucrose gradient centrifugation and cross-linking analyses show that they form oligomers. Mutants of yeast Rtn1p, which no longer localize exclusively to the tubular ER or are even totally inactive in inducing ER tubules, are more mobile and oligomerize less extensively. The mammalian reticulons and DP1 are also relatively immobile and can form oligomers. The conserved reticulon homology domain that includes the two membrane-embedded segments is sufficient for the localization of the reticulons to the tubular ER, as well as for their diffusional immobility and oligomerization. Finally, ATP depletion in both yeast and mammalian cells further decreases the mobilities of the reticulons and DP1. We propose that oligomerization of the reticulons and DP1/Yop1p is important for both their localization to the tubular domains of the ER and for their ability to form tubules.
Figures
References
-
- Staehelin, L. A. (1997) Plant J. 11 1151-1165 - PubMed
-
- Baumann, O., and Walz, B. (2001) Int. Rev. Cytol. 205 149-214 - PubMed
-
- Estrada de Martin, P., Novick, P., and Ferro-Novick, S. (2005) Biochem. Cell Biol. 83 752-761 - PubMed
-
- Shibata, Y., Voeltz, G. K., and Rapoport, T. A. (2006) Cell 126 435-439 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

