Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis
- PMID: 18443023
- PMCID: PMC10802128
- DOI: 10.1001/jama.299.19.jrv80007
Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis
Erratum in
- JAMA. 2008 Sep 17;300(11): 1300
Abstract
Context: Hemoglobin-based blood substitutes (HBBSs) are infusible oxygen-carrying liquids that have long shelf lives, have no need for refrigeration or cross-matching, and are ideal for treating hemorrhagic shock in remote settings. Some trials of HBBSs during the last decade have reported increased risks without clinical benefit.
Objective: To assess the safety of HBBSs in surgical, stroke, and trauma patients.
Data sources: PubMed, EMBASE, and Cochrane Library searches for articles using hemoglobin and blood substitutes from 1980 through March 25, 2008; reviews of Food and Drug Administration (FDA) advisory committee meeting materials; and Internet searches for company press releases.
Study selection: Randomized controlled trials including patients aged 19 years and older receiving HBBSs therapeutically. The database searches yielded 70 trials of which 13 met these criteria; in addition, data from 2 other trials were reported in 2 press releases, and additional data were included in 1 relevant FDA review.
Data extraction: Data on death and myocardial infarction (MI) as outcome variables.
Results: Sixteen trials involving 5 different products and 3711 patients in varied patient populations were identified. A test for heterogeneity of the results of these trials was not significant for either mortality or MI (for both, I2 = 0%, P > or = .60), and data were combined using a fixed-effects model. Overall, there was a statistically significant increase in the risk of death (164 deaths in the HBBS-treated groups and 123 deaths in the control groups; relative risk [RR], 1.30; 95% confidence interval [CI], 1.05-1.61) and risk of MI (59 MIs in the HBBS-treated groups and 16 MIs in the control groups; RR, 2.71; 95% CI, 1.67-4.40) with these HBBSs. Subgroup analysis of these trials indicated the increased risk was not restricted to a particular HBBS or clinical indication.
Conclusion: Based on the available data, use of HBBSs is associated with a significantly increased risk of death and MI.
Figures

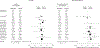

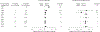
Comment in
-
The future of clinical trials evaluating blood substitutes.JAMA. 2008 May 21;299(19):2324-6. doi: 10.1001/jama.299.19.jed80027. Epub 2008 Apr 28. JAMA. 2008. PMID: 18443022 No abstract available.
-
Hemoglobin-based blood substitutes and risk of myocardial infarction and death.JAMA. 2008 Sep 17;300(11):1295-6; author reply 1298-9. doi: 10.1001/jama.300.11.1295-b. JAMA. 2008. PMID: 18799435 No abstract available.
-
Hemoglobin-based blood substitutes and risk of myocardial infarction and death.JAMA. 2008 Sep 17;300(11):1295; author reply 1298-9. doi: 10.1001/jama.300.11.1295-a. JAMA. 2008. PMID: 18799436 No abstract available.
-
Hemoglobin-based blood substitutes and risk of myocardial infarction and death.JAMA. 2008 Sep 17;300(11):1296-7; author reply 1298-9. doi: 10.1001/jama.300.11.1296-b. JAMA. 2008. PMID: 18799437 No abstract available.
-
Hemoglobin-based blood substitutes and risk of myocardial infarction and death.JAMA. 2008 Sep 17;300(11):1296; author reply 1298-9. doi: 10.1001/jama.300.11.1296-a. JAMA. 2008. PMID: 18799438 No abstract available.
-
Hemoglobin-based blood substitutes and risk of myocardial infarction and death.JAMA. 2008 Sep 17;300(11):1297-8; author reply 1298-9. doi: 10.1001/jama.300.11.1297-b. JAMA. 2008. PMID: 18799439 No abstract available.
-
Hemoglobin-based blood substitutes and risk of myocardial infarction and death.JAMA. 2008 Sep 17;300(11):1297; author reply 1298-9. doi: 10.1001/jama.300.11.1297-a. JAMA. 2008. PMID: 18799440 No abstract available.
-
Incomplete financial disclosure in a study of cell-free hemoglobin-based blood substitutes and risks of myocardial infarction and death.JAMA. 2008 Sep 17;300(11):1300. doi: 10.1001/jama.300.11.1300-a. JAMA. 2008. PMID: 18799441 No abstract available.
-
Review: hemoglobin-based blood substitutes increase mortality and myocardial infarction in surgical, trauma, and stroke settings.ACP J Club. 2008 Nov 18;149(5):4. ACP J Club. 2008. PMID: 19014171 No abstract available.
-
Are Hemoglobin-Based Oxygen Carriers Being Withheld Because of Regulatory Requirement for Equivalence to Packed Red Blood Cells?Am J Ther. 2015 Jul-Aug;22(4):e115-21. doi: 10.1097/MJT.0000000000000009. Am J Ther. 2015. PMID: 25285795 No abstract available.
References
-
- Saxena R, Wijnhoud AD, Carton H, et al. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke. 1999; 30(5):993–996. - PubMed
-
- A single-center study to evaluate the safety and tolerability of hemoglobin-based oxygen carrier-201 (HBOC 201) in trauma subjects (phase II–safety and tolerability). http://clinicaltrials.gov/ct2/show/NCT00301483. Accessed December 10, 2007.
-
- Phase II, open-label study in the catheterization laboratory setting to challenge the concept that HBOC-201 administration might improve myocardial “oxygenation” and myocardial function at the moment of (brief) coronary occlusion. http://clinicaltrials.gov/ct2/show/NCT00479895. Accessed December 10, 2007.
-
- Enhancement of tissue preservation during cardio-pulmonary bypass with HBOC-201 (registry study). http://clinicaltrials.gov/ct2/show/NCT00301535. Accessed December 10, 2007.
-
- A randomized, double-blind, phase III study of the efficacy and safety of an oxygen-carrying plasma expander, Hemospan, compared with Voluven to treat hypotension in patients undergoing primary hip arthroplasty with spinal anesthesia. http://clinicaltrials.gov/ct2/show/NCT00420277. Accessed January 8, 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

