Ydj1 protects nascent protein kinases from degradation and controls the rate of their maturation
- PMID: 18443039
- PMCID: PMC2447146
- DOI: 10.1128/MCB.00543-08
Ydj1 protects nascent protein kinases from degradation and controls the rate of their maturation
Abstract
Ydj1 is a Saccharomyces cerevisiae Hsp40 molecular chaperone that functions with Hsp70 to promote polypeptide folding. We identified Ydj1 as being important for maintaining steady-state levels of protein kinases after screening several chaperones and cochaperones in gene deletion mutant strains. Pulse-chase analyses revealed that a portion of Tpk2 kinase was degraded shortly after synthesis in a ydj1Delta mutant, while the remainder was capable of maturing but with reduced kinetics compared to the wild type. Cdc28 maturation was also delayed in the ydj1Delta mutant strain. Ydj1 protects nascent kinases in different contexts, such as when Hsp90 is inhibited with geldanamycin or when CDC37 is mutated. The protective function of Ydj1 is due partly to its intrinsic chaperone function, but this is minor compared to the protective effect resulting from its interaction with Hsp70. SIS1, a type II Hsp40, was unable to suppress defects in kinase accumulation in the ydj1Delta mutant, suggesting some specificity in Ydj1 chaperone action. However, analysis of chimeric proteins that contained the chaperone modules of Ydj1 or Sis1 indicated that Ydj1 promotes kinase accumulation independently of its client-binding specificity. Our results suggest that Ydj1 can both protect nascent chains against degradation and control the rate of kinase maturation.
Figures
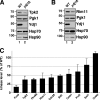
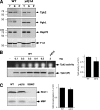


 , ydj1Δ mutant. (C) Pulse-chase analysis of TAP-tagged Cdc28 in wild-type and ydj1Δ cells as described above.
, ydj1Δ mutant. (C) Pulse-chase analysis of TAP-tagged Cdc28 in wild-type and ydj1Δ cells as described above.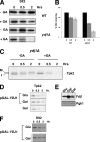


References
-
- Arlander, S. J., S. J. Felts, J. M. Wagner, B. Stensgard, D. O. Toft, and L. M. Karnitz. 2006. Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones. J. Biol. Chem. 2812989-2998. - PubMed
-
- Batkin, M., I. Schvartz, and S. Shaltiel. 2000. Snapping of the carboxyl terminal tail of the catalytic subunit of PKA onto its core: characterization of the sites by mutagenesis. Biochemistry 395366-5373. - PubMed
-
- Bercovich, B., I. Stancovski, A. Mayer, N. Blumenfeld, A. Laszlo, A. L. Schwartz, and A. Ciechanover. 1997. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J. Biol. Chem. 2729002-9010. - PubMed
-
- Brodsky, J. L., J. G. Lawrence, and A. J. Caplan. 1998. Mutations in the cytosolic DnaJ homologue, YDJ1, delay and compromise the efficient translation of heterologous proteins in yeast. Biochemistry 3718045-18055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
