Burkholderia pseudomallei type III secretion system mutants exhibit delayed vacuolar escape phenotypes in RAW 264.7 murine macrophages
- PMID: 18443088
- PMCID: PMC2446725
- DOI: 10.1128/IAI.00263-08
Burkholderia pseudomallei type III secretion system mutants exhibit delayed vacuolar escape phenotypes in RAW 264.7 murine macrophages
Abstract
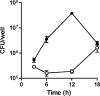
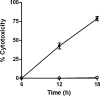
Burkholderia pseudomallei is a facultative intracellular pathogen capable of surviving and replicating within eukaryotic cells. Recent studies have shown that B. pseudomallei Bsa type III secretion system 3 (T3SS-3) mutants exhibit vacuolar escape and replication defects in J774.2 murine macrophages. In the present study, we characterized the interactions of a B. pseudomallei bsaZ mutant with RAW 264.7 murine macrophages. Following uptake, the mutant was found to survive and replicate within infected RAW 264.7 cells over an 18-h period. In addition, high levels of tumor necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6), granulocyte-macrophage colony-stimulating factor (GM-CSF), and RANTES, but not IL-1alpha and IL-1beta, were detected in culture supernatants harvested from infected monolayers. The subcellular location of B. pseudomallei within infected RAW 264.7 cells was determined, and as expected, the bsaZ mutant demonstrated early-vacuolar-escape defects. Interestingly, however, experiments also indicated that this mutant was capable of delayed vacuolar escape. Consistent with this finding, evidence of actin-based motility and multinucleated giant cell formation were observed between 12 and 18 h postinfection. Further studies demonstrated that a triple mutant defective in all three B. pseudomallei T3SSs exhibited the same phenotype as the bsaZ mutant, indicating that functional T3SS-1 and T3SS-2 did not appear to be responsible for the delayed escape phenotype in RAW 264.7 cells. Based upon these findings, it appears that B. pseudomallei may not require T3SS-1, -2, and -3 to facilitate survival, delayed vacuolar escape, and actin-based motility in activated RAW 264.7 macrophages.
Figures



Similar articles
-
An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen.Mol Microbiol. 2002 Nov;46(3):649-59. doi: 10.1046/j.1365-2958.2002.03190.x. Mol Microbiol. 2002. PMID: 12410823
-
Inactivation of Burkholderia pseudomallei bsaQ results in decreased invasion efficiency and delayed escape of bacteria from endocytic vesicles.Arch Microbiol. 2008 Dec;190(6):623-31. doi: 10.1007/s00203-008-0413-3. Epub 2008 Jul 25. Arch Microbiol. 2008. PMID: 18654761
-
Burkholderia pseudomallei Evades Nramp1 (Slc11a1)- and NADPH Oxidase-Mediated Killing in Macrophages and Exhibits Nramp1-Dependent Virulence Gene Expression.Front Cell Infect Microbiol. 2017 Aug 8;7:350. doi: 10.3389/fcimb.2017.00350. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28848712 Free PMC article.
-
Burkholderia mallei cluster 1 type VI secretion mutants exhibit growth and actin polymerization defects in RAW 264.7 murine macrophages.Infect Immun. 2010 Jan;78(1):88-99. doi: 10.1128/IAI.00985-09. Epub 2009 Nov 2. Infect Immun. 2010. PMID: 19884331 Free PMC article.
-
Type III Secretion in the Melioidosis Pathogen Burkholderia pseudomallei.Front Cell Infect Microbiol. 2017 Jun 15;7:255. doi: 10.3389/fcimb.2017.00255. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28664152 Free PMC article. Review.
Cited by
-
Comprehensive genome analysis of a pangolin-associated Paraburkholderia fungorum provides new insights into its secretion systems and virulence.PeerJ. 2020 Sep 3;8:e9733. doi: 10.7717/peerj.9733. eCollection 2020. PeerJ. 2020. PMID: 32953261 Free PMC article.
-
Comparative assessment of the intracellular survival of the Burkholderia pseudomallei bopC mutant.J Microbiol. 2013 Aug;51(4):522-6. doi: 10.1007/s12275-013-2557-3. Epub 2013 Aug 30. J Microbiol. 2013. PMID: 23990305
-
Burkholderia cenocepacia J2315 escapes to the cytosol and actively subverts autophagy in human macrophages.Cell Microbiol. 2014 Mar;16(3):378-95. doi: 10.1111/cmi.12223. Epub 2013 Nov 6. Cell Microbiol. 2014. PMID: 24119232 Free PMC article.
-
Pathogenicity and virulence of Burkholderia pseudomallei.Virulence. 2022 Dec;13(1):1945-1965. doi: 10.1080/21505594.2022.2139063. Virulence. 2022. PMID: 36271712 Free PMC article. Review.
-
Microbial Risks Caused by Livestock Excrement: Current Research Status and Prospects.Microorganisms. 2023 Jul 27;11(8):1897. doi: 10.3390/microorganisms11081897. Microorganisms. 2023. PMID: 37630456 Free PMC article. Review.
References
-
- Attree, O., and I. Attree. 2001. A second type III secretion system in Burkholderia pseudomallei: who is the real culprit? Microbiology 1473197-3199. - PubMed
-
- Boddey, J. A., C. J. Day, C. P. Flegg, R. L. Ulrich, S. R. Stephens, I. R. Beacham, N. A. Morrison, and I. R. Peak. 2007. The bacterial gene lfpA influences the potent induction of calcitonin receptor and osteoclast-related genes in Burkholderia pseudomallei-induced TRAP-positive multinucleated giant cells. Cell. Microbiol. 9514-531. - PubMed
-
- Breitbach, K., K. Rottner, S. Klocke, M. Rohde, A. Jenzora, J. Wehland, and I. Steinmetz. 2003. Actin-based motility of Burkholderia pseudomallei involves the Arp 2/3 complex, but not N-WASP and Ena/VASP proteins. Cell. Microbiol. 5385-393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

