Prion protein attenuates excitotoxicity by inhibiting NMDA receptors
- PMID: 18443219
- PMCID: PMC2364707
- DOI: 10.1083/jcb.200711002
Prion protein attenuates excitotoxicity by inhibiting NMDA receptors
Erratum in
- J Cell Biol. 2009 Jun 15;185(6):1127
Abstract
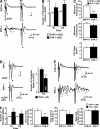
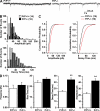
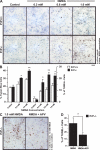
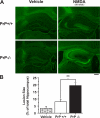
It is well established that misfolded forms of cellular prion protein (PrP [PrP(C)]) are crucial in the genesis and progression of transmissible spongiform encephalitis, whereas the function of native PrP(C) remains incompletely understood. To determine the physiological role of PrP(C), we examine the neurophysiological properties of hippocampal neurons isolated from PrP-null mice. We show that PrP-null mouse neurons exhibit enhanced and drastically prolonged N-methyl-d-aspartate (NMDA)-evoked currents as a result of a functional upregulation of NMDA receptors (NMDARs) containing NR2D subunits. These effects are phenocopied by RNA interference and are rescued upon the overexpression of exogenous PrP(C). The enhanced NMDAR activity results in an increase in neuronal excitability as well as enhanced glutamate excitotoxicity both in vitro and in vivo. Thus, native PrP(C) mediates an important neuroprotective role by virtue of its ability to inhibit NR2D subunits.
Figures


Republished in
-
Prion protein attenuates excitotoxicity by inhibiting NMDA receptors.J Gen Physiol. 2008 Jun;131(6):i5. doi: 10.1085/JGP1316OIA5. J Gen Physiol. 2008. PMID: 18504311
Comment in
-
All quiet on the neuronal front: NMDA receptor inhibition by prion protein.J Cell Biol. 2008 May 5;181(3):407-9. doi: 10.1083/jcb.200803152. Epub 2008 Apr 28. J Cell Biol. 2008. PMID: 18443224 Free PMC article.
References
-
- Altier, C., H. Khosravani, R.M. Evans, S. Hameed, J.B. Peloquin, B.A. Vartian, L. Chen, A.M. Beedle, S.S. Ferguson, A. Mezghrani, et al. 2006. ORL1 receptor-mediated internalization of N-type calcium channels. Nat. Neurosci. 9:31–40. - PubMed
-
- Brown, D.R. 2001. Prion and prejudice: normal protein and the synapse. Trends Neurosci. 24:85–90. - PubMed
-
- Brown, D.R., J.W. Herms, B. Schmidt, and H.A. Kretzschmar. 1997. PrP and beta-amyloid fragments activate different neurotoxic mechanisms in cultured mouse cells. Eur. J. Neurosci. 9:1162–1169. - PubMed
-
- Bueler, H., M. Fischer, Y. Lang, H. Bluethmann, H.P. Lipp, S.J. DeArmond, S.B. Prusiner, M. Aguet, and C. Weissmann. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 356:577–582. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

