Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance
- PMID: 18443281
- PMCID: PMC2373349
- DOI: 10.1073/pnas.0802690105
Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance
Abstract
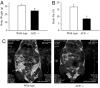
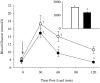
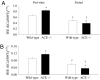
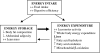
In addition to its role in the storage of fat, adipose tissue acts as an endocrine organ, and it contains a functional renin-angiotensin system (RAS). Angiotensin-converting enzyme (ACE) plays a key role in the RAS by converting angiotensin I to the bioactive peptide angiotensin II (Ang II). In the present study, the effect of targeting the RAS in body energy homeostasis and glucose tolerance was determined in homozygous mice in which the gene for ACE had been deleted (ACE(-/-)) and compared with wild-type littermates. Compared with wild-type littermates, ACE(-/-) mice had lower body weight and a lower proportion of body fat, especially in the abdomen. ACE(-/-) mice had greater fed-state total energy expenditure (TEE) and resting energy expenditure (REE) than wild-type littermates. There were pronounced increases in gene expression of enzymes related to lipolysis and fatty acid oxidation (lipoprotein lipase, carnitine palmitoyl transferase, long-chain acetyl CoA dehydrogenase) in the liver of ACE(-/-) mice and also lower plasma leptin. In contrast, no differences were detected in daily food intake, activity, fed-state plasma lipids, or proportion of fat excreted in fecal matter. In conclusion, the reduction in ACE activity is associated with a decreased accumulation of body fat, especially in abdominal fat depots. The decreased body fat in ACE(-/-) mice is independent of food intake and appears to be due to a high energy expenditure related to increased metabolism of fatty acids in the liver, with the additional effect of increased glucose tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
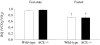

References
-
- Dustan HP. Physiologic regulation of arterial blood pressure. Hypertension. 1982;4:62–67. - PubMed
-
- Thurman JM, Schrier RW. Comparative effects of ACE inhibitors and ARBs on blood pressure and the kidney. Am J Med. 2003;114:588–598. - PubMed
-
- Tarjan E, Denton DA, McBurnie MI, Weisinger RS. Water and Na intake of wild and New Zealand rabbits. Peptides. 1988;9:677–679. - PubMed
-
- Weisinger RS, et al. The problem of obesity: Is there a role for antagonists of the renin-angiotensin system? Asia Pac J Clin Nutr. 2007;16(Suppl 1):359–367. - PubMed
-
- Hainault I, et al. Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese Zucker rat. Am J Physiol Endocrinol Metab. 2002;282:E59–E66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

