Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice
- PMID: 18443602
- PMCID: PMC2782410
- DOI: 10.1038/mt.2008.81
Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice
Abstract
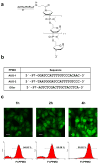
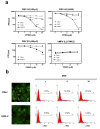
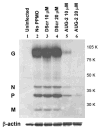
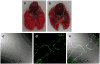
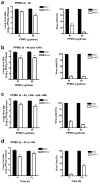
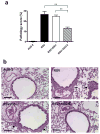
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infection in infants, young children, and high-risk adults. Currently, there is no vaccine to prevent RSV infection, and the available therapeutic agents are of limited utility. Peptide-conjugated phosphorodiamidate morpholino oligomers (PPMOs) are a class of antisense agents that can enter cells readily and interfere with viral protein expression through steric blocking of complementary RNA. Two antisense PPMOs, designed to target sequence that includes the 5'-terminal region and translation start-site region of RSV L mRNA, were tested for anti-RSV activity in cultures of two human-airway cell lines. Both PPMOs showed minimal cytotoxicity and one of them, (AUG-2), reduced viral titers by >2.0 log(10). Intranasal (i.n.) treatment of BALB/c mice with AUG-2 PPMO before the RSV inoculation produced a reduction in viral titer of 1.2 log(10) in lung tissue at day 5 postinfection (p.i.), and attenuated pulmonary inflammation at day 7 postinfection. These data show that the AUG-2 PPMO possesses potent anti-RSV activity and is worthy of further investigation as a candidate for potential therapeutic application.
Figures


References
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. - PubMed
-
- Marquardt ED. Cost of ribavirin therapy for respiratory syncytial virus infection. J Pediatr. 1995;126:847. - PubMed
-
- Crotty S, Andino R. Implications of high RNA virus mutation rates: lethal mutagenesis and the antiviral drug ribavirin. Microbes Infect. 2002;4:1301–1307. - PubMed
-
- Crotty S, Cameron C, Andino R. Ribavirin’s antiviral mechanism of action: lethal mutagenesis? J Mol Med. 2002;80:86–95. - PubMed
-
- Prince GA. An update on respiratory syncytial virus antiviral agents. Expert Opin Investig Drugs. 2001;10:297–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

