Intermediate filament cytoskeleton of the liver in health and disease
- PMID: 18443813
- PMCID: PMC2386529
- DOI: 10.1007/s00418-008-0431-x
Intermediate filament cytoskeleton of the liver in health and disease
Abstract
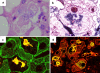
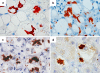
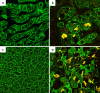
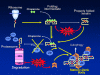
Intermediate filaments (IFs) represent the largest cytoskeletal gene family comprising approximately 70 genes expressed in tissue specific manner. In addition to scaffolding function, they form complex signaling platforms and interact with various kinases, adaptor, and apoptotic proteins. IFs are established cytoprotectants and IF variants are associated with >30 human diseases. Furthermore, IF-containing inclusion bodies are characteristic features of several neurodegenerative, muscular, and other disorders. Acidic (type I) and basic keratins (type II) build obligatory type I and type II heteropolymers and are expressed in epithelial cells. Adult hepatocytes contain K8 and K18 as their only cytoplasmic IF pair, whereas cholangiocytes express K7 and K19 in addition. K8/K18-deficient animals exhibit a marked susceptibility to various toxic agents and Fas-induced apoptosis. In humans, K8/K18 variants predispose to development of end-stage liver disease and acute liver failure (ALF). K8/K18 variants also associate with development of liver fibrosis in patients with chronic hepatitis C. Mallory-Denk bodies (MDBs) are protein aggregates consisting of ubiquitinated K8/K18, chaperones and sequestosome1/p62 (p62) as their major constituents. MDBs are found in various liver diseases including alcoholic and non-alcoholic steatohepatitis and can be formed in mice by feeding hepatotoxic substances griseofulvin and 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC). MDBs also arise in cell culture after transfection with K8/K18, ubiquitin, and p62. Major factors that determine MDB formation in vivo are the type of stress (with oxidative stress as a major player), the extent of stress-induced protein misfolding and resulting chaperone, proteasome and autophagy overload, keratin 8 excess, transglutaminase activation with transamidation of keratin 8 and p62 upregulation.
Figures
Similar articles
-
The genetic background modulates susceptibility to mouse liver Mallory-Denk body formation and liver injury.Hepatology. 2008 Sep;48(3):943-52. doi: 10.1002/hep.22436. Hepatology. 2008. PMID: 18697208
-
From Mallory to Mallory-Denk bodies: what, how and why?Exp Cell Res. 2007 Jun 10;313(10):2033-49. doi: 10.1016/j.yexcr.2007.04.024. Epub 2007 Apr 27. Exp Cell Res. 2007. PMID: 17531973 Review.
-
Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies.Hepatology. 2007 Nov;46(5):1639-49. doi: 10.1002/hep.21976. Hepatology. 2007. PMID: 17969036 Review.
-
Keratins 8 and 18 are type II acute-phase responsive genes overexpressed in human liver disease.Liver Int. 2015 Apr;35(4):1203-12. doi: 10.1111/liv.12608. Epub 2014 Jun 26. Liver Int. 2015. PMID: 24930437
-
Mallory-Denk-bodies: lessons from keratin-containing hepatic inclusion bodies.Biochim Biophys Acta. 2008 Dec;1782(12):764-74. doi: 10.1016/j.bbadis.2008.08.008. Epub 2008 Sep 6. Biochim Biophys Acta. 2008. PMID: 18805482 Review.
Cited by
-
Keratin 8/18 Regulate the Akt Signaling Pathway.Int J Mol Sci. 2021 Aug 26;22(17):9227. doi: 10.3390/ijms22179227. Int J Mol Sci. 2021. PMID: 34502133 Free PMC article.
-
Development of a diagnostic nomogram for alpha-fetoprotein-negative hepatocellular carcinoma based on serological biomarkers.World J Gastrointest Oncol. 2024 Jun 15;16(6):2463-2475. doi: 10.4251/wjgo.v16.i6.2463. World J Gastrointest Oncol. 2024. PMID: 38994169 Free PMC article.
-
The role of keratins in the digestive system: lessons from transgenic mouse models.Histochem Cell Biol. 2018 Oct;150(4):351-359. doi: 10.1007/s00418-018-1695-4. Epub 2018 Jul 24. Histochem Cell Biol. 2018. PMID: 30039330 Review.
-
Visceral Obesity and Cytokeratin-18 Antigens as Early Biomarkers of Liver Damage.Int J Mol Sci. 2023 Jun 29;24(13):10885. doi: 10.3390/ijms241310885. Int J Mol Sci. 2023. PMID: 37446065 Free PMC article.
-
Keratin 18, Apoptosis, and Liver Disease in Children.Curr Pediatr Rev. 2011 Nov;7(4):310-315. doi: 10.2174/157339611796892364. Curr Pediatr Rev. 2011. PMID: 25346653 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC19321', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC19321/'}, {'type': 'PubMed', 'value': '8990203', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8990203/'}]}
- Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K (1997) Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA 94:298–303 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15483602', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15483602/'}]}
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431:805–810 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15486927', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15486927/'}]}
- Bantel H, Lugering A, Heidemann J, Volkmann X, Poremba C, Strassburg CP, Manns MP, Schulze-Osthoff K (2004) Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology 40:1078–1087 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15234234', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15234234/'}]}
- Barak V, Goike H, Panaretakis KW, Einarsson R (2004) Clinical utility of cytokeratins as tumor markers. Clin Biochem 37:529–540 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11170786', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11170786/'}]}
- Bardag-Gorce F, French BA, Lue YH, Nguyen V, Wan YJ, French SW (2001) Mallory bodies formed in proteasome-depleted hepatocytes: an immunohistochemical study. Exp Mol Pathol 70:7–18 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

