Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2
- PMID: 18444786
- PMCID: PMC2656366
- DOI: 10.1089/hum.2007.0171
Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2
Abstract
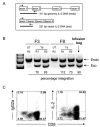
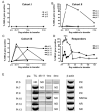
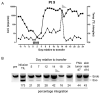
Adoptive cell transfer of tumor-infiltrating lymphocytes (TILs) after lymphodepletion mediates regression in 50% of patients with metastatic melanoma. In vivo persistence and telomere length of the transferred cells correlate with antitumor response. In an attempt to prolong the in vivo survival of the transferred cells, TILs were genetically engineered to produce interleukin (IL)-2. In vitro, these transduced TILs secreted IL-2 while retaining tumor specificity and exhibited prolonged survival after IL-2 withdrawal. In a phase I/II clinical trial, seven evaluable patients received transduced TILs and one patient experienced a partial response associated with in vivo persistence of IL-2-transduced TILs in circulating lymphocytes. An additional five patients received transduced TILs in conjunction with IL-2 administration. Persistence of IL-2-transduced TILs was observed in three patients, including one partial responder. The transgene DNA as well as vector-derived IL2 mRNA could be detected for 4 months in responding patients. The low response rate in this trial was possibly due to a reduction in telomere length in cells as a result of prolonged in vitro culture. In this study, insertion of the IL-2 gene into antitumor TILs increased their ability to survive after IL-2 withdrawal in vitro but did not increase their in vivo persistence or clinical effectiveness.
Figures




References
-
- ANTONY PA, PICCIRILLO CA, AKPINARLI A, FINKELSTEIN SE, SPEISS PJ, SURMAN DR, PALMER DC, CHAN CC, KLEBANOFF CA, OVERWIJK WW, ROSENBERG SA, RESTIFO NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 2005;174:2591–2601. - PMC - PubMed
-
- CAVALIERI S, CAZZANIGA S, GEUNA M, MAGNANI Z, BORDIGNON C, NALDINI L, BONINI C. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. - PubMed
-
- DUDLEY ME, WUNDERLICH JR, ROBBINS PF, YANG JC, HWU P, SCHWARTZENTRUBER DJ, TOPALIAN SL, SHERRY R, RESTIFO NP, HUBICKI AM, ROBINSON MR, RAFFELD M, DURAY P, SEIPP CA, ROGERS-FREEZER L, MORTON KE, MAVROUKAKIS SA, WHITE DE, ROSENBERG SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002a;298:850–854. - PMC - PubMed
-
- DUDLEY ME, WUNDERLICH JR, YANG JC, HWU P, SCHWARTZENTRUBER DJ, TOPALIAN SL, SHERRY RM, MARINCOLA FM, LEITMAN SF, SEIPP CA, ROGERS-FREEZER L, MORTON KE, NAHVI A, MAVROUKAKIS SA, WHITE DE, ROSENBERG SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J. Immunother. 2002b;25:243–251. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

