Safety and immunogenicity of trivalent inactivated influenza vaccine in infants
- PMID: 18444800
- PMCID: PMC3773726
- DOI: 10.1086/587643
Safety and immunogenicity of trivalent inactivated influenza vaccine in infants
Abstract
Background: Trivalent inactivated influenza vaccine (TIV) is not licensed for use in infants <6 months old, the group with the highest influenza hospitalization rates among children.
Methods: In this prospective, open-label study, 2 doses of TIV were administered to healthy infants aged 10-22 weeks. Adverse reactions were assessed, and hemagglutination inhibition (HAI) antibody titers were determined. Weekly telephone surveillance for influenza-like illness was conducted during the influenza season.
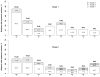
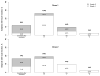
Results: A total of 42 infants were enrolled and completed the study. Mild local and systemic reactions were noted. In the first season (2004-2005), postvaccination HAI titers >1:32 were noted for 31.6%, 47.4%, and 21.1% of 19 subjects for H1N1, H3N2, and B strains included in the vaccine, respectively. In the second season (2005-2006), postvaccination HAI titers >1:32 were seen in 45.5%, 59.1%, and 0% of 23 subjects for H1N1, H3N2, and B strains included in the vaccine, respectively. Infants who were seronegative before vaccination (titers <1:8) were significantly more likely to have a 4-fold rise in antibody titer after vaccination, compared with infants who had prevaccination titers >1:8 (P<.001).
Conclusion: Two doses of TIV were found to be safe and moderately immunogenic against some influenza strains. The presence of preexisting maternally derived antibody was associated with significantly lower seroresponse rates to vaccination. Whether vaccination with TIV will prevent influenza in these young children remains to be determined.
Conflict of interest statement
Potential conflicts of interest: N.B.H. receives grant support from MedImmune and Sanofi Pasteur. K.M.E. receives grant support from Sanofi Pasteur, MedImmune, VaxGen, and Merck and serves as a consultant to Wyeth, MedImmune, and PATH. P.F.W. receives grant support from MedImmune, GlaxoSmithKline, and Merck and serves as a consultant to Alphavax, MedImmune, and Novartis. M.A.G. receives grant support from Wyeth, Merck, Sanofi Pasteur.
Figures
References
-
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. - PubMed
-
- Glezen WP, Paredes A, Taber LH. Influenza in children: relationship to other respiratory agents. JAMA. 1980;243:1345–9. - PubMed
-
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–31. - PubMed
-
- Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

