Lsa21, a novel leptospiral protein binding adhesive matrix molecules and present during human infection
- PMID: 18445272
- PMCID: PMC2386478
- DOI: 10.1186/1471-2180-8-70
Lsa21, a novel leptospiral protein binding adhesive matrix molecules and present during human infection
Abstract
Background: It has been well documented over past decades that interaction of pathogens with the extracellular matrix (ECM) plays a primary role in host cell attachment and invasion. Adherence to host tissues is mediated by surface-exposed proteins expressed by the microorganisms during infection. The mechanisms by which pathogenic leptospires invade and colonize the host remain poorly understood since few virulence factors contributing to the pathogenesis of the disease have been identified. Whole-genome sequencing analysis of L. interrogans allowed identification of a repertoire of putative leptospiral surface proteins.
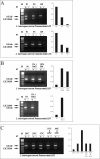
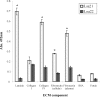
Results: Here, we report the identification and characterization of a new leptospiral protein that exhibits extracellular matrix-binding properties, called as Lsa21 (leptospiral surface adhesin, 21 kDa). Compatible with its role in adhesion, the protein was shown to be surface-exposed by indirect immunofluorescence. Attachment of Lsa21 to laminin, collagen IV, and plasma fibronectin was specific and dose dependent. Laminin oxidation by sodium metaperiodate reduced the protein-laminin interaction in a concentration-dependent manner, indicating that laminin sugar moieties are crucial for this interaction. The gene coding for Lsa21 is present in pathogenic strains belonging to the L. interrogans species but was not found in the saprophytic L. biflexa serovar Patoc strain Patoc 1. Loss of gene expression occurs upon culture attenuation of pathogenic strains. Environmental factors such as osmolarity and temperature affect Lsa21 expression at the transcriptional level. Moreover, anti-Lsa21 serum labeled liver and kidney tissues of human fatal cases of leptospirosis.
Conclusion: Our data suggest a role of Lsa21 in the pathogenesis of leptospirosis.
Figures


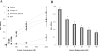

References
-
- Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. 2. Melbourne, Australia: MediSci; 1999.
-
- Plank R, Dean D. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes and infection/Institut Pasteur. 2000;2:1265–1276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

