PAI1 stimulates assembly of the fibronectin matrix in osteosarcoma cells through crosstalk between the alphavbeta5 and alpha5beta1 integrins
- PMID: 18445685
- PMCID: PMC2535923
- DOI: 10.1242/jcs.020149
PAI1 stimulates assembly of the fibronectin matrix in osteosarcoma cells through crosstalk between the alphavbeta5 and alpha5beta1 integrins
Abstract
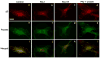
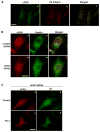
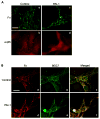
The plasminogen activation system regulates matrix remodeling through both proteolytic and non-proteolytic mechanisms. Studies were undertaken to determine the effects of the plasminogen activator inhibitor 1 (PAI1) on the assembly of the fibronectin matrix. The addition of PAI1 to MG-63 cells caused a 1.5- to threefold increase in the rate of fibronectin matrix assembly which was associated with an increase in beta integrin activation. PAI1 treatment led to a marked decrease in focal contacts and stress fibers, whereas tensin-containing matrix contacts remained unaffected. The effects of PAI1 on matrix assembly were independent of both urokinase-type plasminogen activator (uPA) and urokinase-type plasminogen activator receptor (uPAR), indicating that the stimulation of matrix assembly by PAI1 does not depend on its anti-proteolytic activity or on the association of uPAR with integrin receptors. Antagonists of the alphavbeta5 integrin mimicked the effect of PAI1 on cell morphology and fibronectin matrix deposition, indicating that stimulation of matrix assembly by PAI1 required disruption of the interaction between the alphavbeta5 integrin and vitronectin. Consistent with this conclusion, the Q123K PAI1 mutant which does not bind vitronectin had no effect on matrix assembly. Our data identify PAI1 as a novel regulator of fibronectin matrix assembly, and indicate that this regulation occurs through a previously undescribed crosstalk between the alphavbeta5 and alpha5beta1 integrins.
Figures

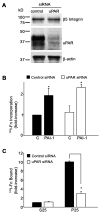
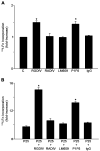
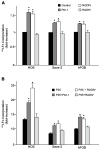
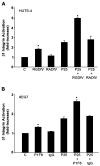


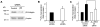
References
-
- Brenner KA, Corbett SA, Schwarzbauer J. Regulation of fibronectin matrix assembly by activated Ras in transformed cells. Oncogene. 2000;19:3156–3163. - PubMed
-
- Chambers SK, Irvins CM, Carcamgiu ML. Plasminogen activator inhibitor-1 is an independent poor prognostic factor for survival in advanced stage epithelial ovarian cancer patients. Int J Cancer. 1998;79:449–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

