Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity
- PMID: 18445751
- PMCID: PMC3751035
- DOI: 10.1152/ajpendo.90281.2008
Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity
Abstract
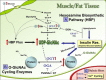
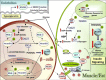
O-linked beta-N-acetylglucosamine (O-GlcNAc) is a dynamic posttranslational modification that, analogous to phosphorylation, cycles on and off serine and/or threonine hydroxyl groups. Cycling of O-GlcNAc is regulated by the concerted actions of O-GlcNAc transferase and O-GlcNAcase. GlcNAcylation is a nutrient/stress-sensitive modification that regulates proteins involved in a wide array of biological processes, including transcription, signaling, and metabolism. GlcNAcylation is involved in the etiology of glucose toxicity and chronic hyperglycemia-induced insulin resistance, a major hallmark of type 2 diabetes. Several reports demonstrate a strong positive correlation between GlcNAcylation and the development of insulin resistance. However, recent studies suggest that inhibiting GlcNAcylation does not prevent hyperglycemia-induced insulin resistance, suggesting that other mechanisms must also be involved. To date, proteomic analyses have identified more than 600 GlcNAcylated proteins in diverse functional classes. However, O-GlcNAc sites have been mapped on only a small percentage (<15%) of these proteins, most of which were isolated from brain or spinal cord tissue and not from other metabolically relevant tissues. Mapping the sites of GlcNAcylation is not only necessary to elucidate the complex cross-talk between GlcNAcylation and phosphorylation but is also key to the design of site-specific mutational studies and necessary for the generation of site-specific antibodies, both of which will help further decipher O-GlcNAc's functional roles. Recent technical advances in O-GlcNAc site-mapping methods should now finally allow for a much-needed increase in site-specific analyses to address the functional significance of O-GlcNAc in insulin resistance and glucose toxicity as well as other major biological processes.
Figures

References
-
- AkimotoYAkimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, Ohara-Imaizumi M, Nishiwaki C, Nagamatsu S, Hirano H, Kawakami H. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology 17: 127–140, 2007. - PubMed
-
- AkimotoYAkimoto Y, Kreppel LK, Hirano H, Hart GW. Localization of the O-linked N-acetylglucosamine transferase in rat pancreas. Diabetes 48: 2407–2413, 1999. - PubMed
-
- AlbertTAlbert T, Urlbauer B, Kohlhuber F, Hammersen B, Eick D. Ongoing mutations in the N-terminal domain of c-Myc affect transactivation in Burkitt's lymphoma cell lines. Oncogene 9: 759–763, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

