Prevention of TNF-induced necrotic cell death by rottlerin through a Nox1 NADPH oxidase
- PMID: 18446057
- PMCID: PMC2679309
- DOI: 10.3858/emm.2008.40.2.186
Prevention of TNF-induced necrotic cell death by rottlerin through a Nox1 NADPH oxidase
Abstract
Previous studies have demonstrated that rottlerin, a specific PKCdelta inhibitor, potentiates death receptor- mediated apoptosis through a cytochrome c-dependent or -independent pathway. However, its ability to regulate necrotic cell death, as well as the underlying mechanism, remains unknown. We found that in murine fibrosarcoma L929 cells, treatment with rottlerin protected the cells against TNF-induced necrosis, whereas it sensitized the cells to apoptosis induced by co-treatment with Hsp90 inhibitor geldanamycin and TNF, in a manner independent of its ability to inhibit PKC-delta. TNF treatment induced rapid accumulation of mitochondrial superoxide (O2-) through the Nox1 NADPH oxidase when cells undergo necrosis. Moreover, pretreatment with rottlerin failed to induce the GTP-bound form of small GTPase Rac1 by TNF treatment, and subsequently suppressed mitochondrial O2- production and poly(ADP-ribose) polymerase activation, thus inhibiting necrotic cell death. Therefore, our study suggests that Nox1 NADPH oxidase is a new molecular target for anti-necrotic activity of rottlerin upon death-receptor ligation.
Figures

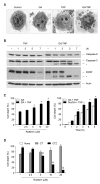
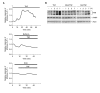

References
-
- Bánfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. - PubMed
-
- Byun HS, Park KA, Won M, Yang KJ, Shin S, Piao L, Kwak JY, Lee ZW, Park J, Seok JH, Liu ZG, Hur GM. Phorbol 12-myristate 13-acetate protects against tumor necrosis factor (TNF)-induced necrotic cell death by modulating the recruitment of TNF receptor 1-associated death domain and receptor-interacting protein into the TNF receptor 1 signaling complex: Implication for the regulatory role of protein kinase C. Mol Pharmacol. 2006;70:1099–1108. - PubMed
-
- Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. - PubMed
-
- Fas SC, Fritzsching B, Suri-Payer E, Krammer PH. Death receptor signaling and its function in the immune system. Curr Dir Autoimmun. 2006;9:1–17. - PubMed
-
- Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
