Calmodulin activation by calcium transients in the postsynaptic density of dendritic spines
- PMID: 18446197
- PMCID: PMC2312328
- DOI: 10.1371/journal.pone.0002045
Calmodulin activation by calcium transients in the postsynaptic density of dendritic spines
Abstract
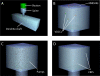
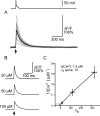
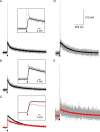
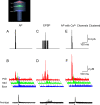
The entry of calcium into dendritic spines can trigger a sequence of biochemical reactions that begins with the activation of calmodulin (CaM) and ends with long-term changes to synaptic strengths. The degree of activation of CaM can depend on highly local elevations in the concentration of calcium and the duration of transient increases in calcium concentration. Accurate measurement of these local changes in calcium is difficult because the spaces are so small and the numbers of molecules are so low. We have therefore developed a Monte Carlo model of intracellular calcium dynamics within the spine that included calcium binding proteins, calcium transporters and ion channels activated by voltage and glutamate binding. The model reproduced optical recordings using calcium indicator dyes and showed that without the dye the free intracellular calcium concentration transient was much higher than predicted from the fluorescent signal. Excitatory postsynaptic potentials induced large, long-lasting calcium gradients across the postsynaptic density, which activated CaM. When glutamate was released at the synapse 10 ms before an action potential occurred, simulating activity patterns that strengthen hippocampal synapses, the calcium gradient and activation of CaM in the postsynaptic density were much greater than when the order was reversed, a condition that decreases synaptic strengths, suggesting a possible mechanism underlying the induction of long-term changes in synaptic strength. The spatial and temporal mechanisms for selectivity in CaM activation demonstrated here could be used in other signaling pathways.
Conflict of interest statement
Figures


References
-
- Kennedy MB. Regulation of neuronal function by calcium. Trends Neurosci. 1989;12:417–420. - PubMed
-
- Sabatini B, Oertner TG, Svoboda K. The life cycle of Ca2+ ions in dendritic spines. Neuron. 2002;33:439–452. - PubMed
-
- Svoboda K, Tank DW, Denk W. Direct measurement of coupling between dendritic spines and shafts. Science. 1996;272(5262):716–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

