Cyanogenic pseudomonads influence multitrophic interactions in the rhizosphere
- PMID: 18446201
- PMCID: PMC2315799
- DOI: 10.1371/journal.pone.0002073
Cyanogenic pseudomonads influence multitrophic interactions in the rhizosphere
Abstract
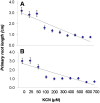
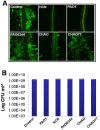
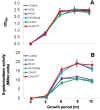
In the rhizosphere, plant roots cope with both pathogenic and beneficial bacterial interactions. The exometabolite production in certain bacterial species may regulate root growth and other root-microbe interactions in the rhizosphere. Here, we elucidated the role of cyanide production in pseudomonad virulence affecting plant root growth and other rhizospheric processes. Exposure of Arabidopsis thaliana Col-0 seedlings to both direct (with KCN) and indirect forms of cyanide from different pseudomonad strains caused significant inhibition of primary root growth. Further, we report that this growth inhibition was caused by the suppression of an auxin responsive gene, specifically at the root tip region by pseudomonad cyanogenesis. Additionally, pseudomonad cyanogenesis also affected other beneficial rhizospheric processes such as Bacillus subtilis colonization by biofilm formation on A. thaliana Col-0 roots. The effect of cyanogenesis on B. subtilis biofilm formation was further established by the down regulation of important B. subtilis biofilm operons epsA and yqxM. Our results show, the functional significance of pseudomonad cyanogenesis in regulating multitrophic rhizospheric interactions.
Conflict of interest statement
Figures




References
-
- Rodgers PB, Knowles CJ. Cyanide production and degradation during growth of Chromobacterium violaceum. . Journal of General Microbiology. 1978;108:261–267.
-
- Castric PA. The metabolism of hydrogen cyanide by bacteria. In: Vennesland B, Conn EE, Knowles CJ, Westley J, Wissing F, editors. Cyanide in biology. London: Academic; 1981. pp. 233–261.
-
- Vennesland B, Pistorius EK, Gewitz HS. HCN production by microalgae. In: Vennesland B, Conn EE, Knowles CJ, Westley J, Wissing F, editors. Cyanide in biology. London: Academic; 1981. pp. 349–362.
-
- Knowles CJ, Bunch AW. Microbial cyanide metabolism. Advances in Microbial Physiology. 1986;27:73–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

