NO dioxygenase activity in hemoglobins is ubiquitous in vitro, but limited by reduction in vivo
- PMID: 18446211
- PMCID: PMC2323109
- DOI: 10.1371/journal.pone.0002039
NO dioxygenase activity in hemoglobins is ubiquitous in vitro, but limited by reduction in vivo
Abstract
Genomics has produced hundreds of new hemoglobin sequences with examples in nearly every living organism. Structural and biochemical characterizations of many recombinant proteins reveal reactions, like oxygen binding and NO dioxygenation, that appear general to the hemoglobin superfamily regardless of whether they are related to physiological function. Despite considerable attention to "hexacoordinate" hemoglobins, which are found in nearly every plant and animal, no clear physiological role(s) has been assigned to them in any species. One popular and relevant hypothesis for their function is protection against NO. Here we have tested a comprehensive representation of hexacoordinate hemoglobins from plants (rice hemoglobin), animals (neuroglobin and cytoglobin), and bacteria (Synechocystis hemoglobin) for their abilities to scavenge NO compared to myoglobin. Our experiments include in vitro comparisons of NO dioxygenation, ferric NO binding, NO-induced reduction, NO scavenging with an artificial reduction system, and the ability to substitute for a known NO scavenger (flavohemoglobin) in E. coli. We conclude that none of these tests reveal any distinguishing predisposition toward a role in NO scavenging for the hxHbs, but that any hemoglobin could likely serve this role in the presence of a mechanism for heme iron re-reduction. Hence, future research to test the role of Hbs in NO scavenging would benefit more from the identification of cognate reductases than from in vitro analysis of NO and O(2) binding.
Conflict of interest statement
Figures
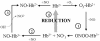
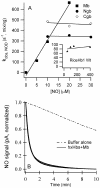


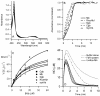

References
-
- Wittenberg JB. Myoglobin-facilitated diffusion of oxygen. J Gen Phys. 1965;49:57–74. - PubMed
-
- Antonini E, Brunori M. Amsterdam: North-Holland Publishing Company; 1971. Hemoglobin and Myoglobin in their Reactions with Ligands; Neuberger A, Tatum EL, editors.
-
- Antonini E. Interrelationship Between Structure and Function in Hemoglobin and Myoglobin. Physiol Rev. 1965;45:123–170. - PubMed
-
- Jia L, Bonaventura C, Bonaventura J, Stamler J. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. - PubMed
-
- Doherty D, Doyle MP, Curry SR, Vali R, Fattor T, et al. The Rate of Reaction with NO Determines the Hypertensive Effect of Cell-free Hemoglobin. Nature Biotech. 1998;16:672–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

