Muscle dystroglycan organizes the postsynapse and regulates presynaptic neurotransmitter release at the Drosophila neuromuscular junction
- PMID: 18446215
- PMCID: PMC2323113
- DOI: 10.1371/journal.pone.0002084
Muscle dystroglycan organizes the postsynapse and regulates presynaptic neurotransmitter release at the Drosophila neuromuscular junction
Abstract
Background: The Dystrophin-glycoprotein complex (DGC) comprises dystrophin, dystroglycan, sarcoglycan, dystrobrevin and syntrophin subunits. In muscle fibers, it is thought to provide an essential mechanical link between the intracellular cytoskeleton and the extracellular matrix and to protect the sarcolemma during muscle contraction. Mutations affecting the DGC cause muscular dystrophies. Most members of the DGC are also concentrated at the neuromuscular junction (NMJ), where their deficiency is often associated with NMJ structural defects. Hence, synaptic dysfunction may also intervene in the pathology of dystrophic muscles. Dystroglycan is a central component of the DGC because it establishes a link between the extracellular matrix and Dystrophin. In this study, we focused on the synaptic role of Dystroglycan (Dg) in Drosophila.
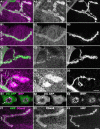
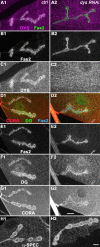
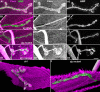
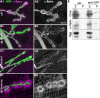
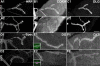
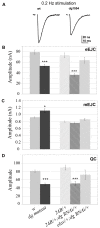
Methodology/principal findings: We show that Dg was concentrated postsynaptically at the glutamatergic NMJ, where, like in vertebrates, it controls the concentration of synaptic Laminin and Dystrophin homologues. We also found that synaptic Dg controlled the amount of postsynaptic 4.1 protein Coracle and alpha-Spectrin, as well as the relative subunit composition of glutamate receptors. In addition, both Dystrophin and Coracle were required for normal Dg concentration at the synapse. In electrophysiological recordings, loss of postsynaptic Dg did not affect postsynaptic response, but, surprisingly, led to a decrease in glutamate release from the presynaptic site.
Conclusion/significance: Altogether, our study illustrates a conservation of DGC composition and interactions between Drosophila and vertebrates at the synapse, highlights new proteins associated with this complex and suggests an unsuspected trans-synaptic function of Dg.
Conflict of interest statement
Figures

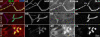

References
-
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, et al. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. - PubMed
-
- Ibraghimov-Beskrovnaya O, Milatovich A, Ozcelik T, Yang B, Koepnick K, et al. Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum Mol Genet. 1993;2:1651–1657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

