The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis
- PMID: 18446225
- PMCID: PMC2323576
- DOI: 10.1371/journal.pone.0002068
The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis
Abstract
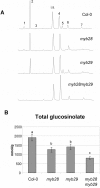
Aliphatic glucosinolates are compounds which occur in high concentrations in Arabidopsis thaliana and other Brassicaceae species. They are important for the resistance of the plant to pest insects. Previously, the biosynthesis of these compounds was shown to be regulated by transcription factors MYB28 and MYB29. We now show that MYB28 and MYB29 are partially redundant, but in the absence of both, the synthesis of all aliphatic glucosinolates is blocked. Untargeted and targeted biochemical analyses of leaf metabolites showed that differences between single and double knock-out mutants and wild type plants were restricted to glucosinolates. Biosynthesis of long-chain aliphatic glucosinolates was blocked by the myb28 mutation, while short-chain aliphatic glucosinolates were reduced by about 50% in both the myb28 and the myb29 single mutants. Most remarkably, all aliphatic glucosinolates were completely absent in the double mutant. Expression of glucosinolate biosynthetic genes was slightly but significantly reduced by the single myb mutations, while the double mutation resulted in a drastic decrease in expression of these genes. Since the myb28myb29 double mutant is the first Arabidopsis genotype without any aliphatic glucosinolates, we used it to establish the relevance of aliphatic glucosinolate biosynthesis to herbivory by larvae of the lepidopteran insect Mamestra brassicae. Plant damage correlated inversely to the levels of aliphatic glucosinolates observed in those plants: Larval weight gain was 2.6 fold higher on the double myb28myb29 mutant completely lacking aliphatic glucosinolates and 1.8 higher on the single mutants with intermediate levels of aliphatic glucosinolates compared to wild type plants.
Conflict of interest statement
Figures







References
-
- Kliebenstein DJ. Secondary metabolites and plant/environment interactions: a view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004;27:675–684.
-
- Wittstock U, Kliebenstein DJ, Lambrix V, Reichelt M, Gershenzon J. Glucosinolate hydrolysis and its impact on geenralist and specialist insect herbivores. In: Romeo JT, editor. Integrative Phytochemistry: from Ethnobotany to Molecular Ecology. Amsterdam: Pergamon; 2003. pp. 101–125.
-
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochem. 2001;56:5–51. - PubMed
-
- Kelly PJ, Bones A, Rossiter JT. Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta. 1998;206:370–377. - PubMed
-
- Agrawal AA, Kurashige NS. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J Chem Ecol. 2003;29:1403–1415. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

