Attomolar detection of botulinum toxin type A in complex biological matrices
- PMID: 18446228
- PMCID: PMC2323579
- DOI: 10.1371/journal.pone.0002041
Attomolar detection of botulinum toxin type A in complex biological matrices
Abstract
Background: A highly sensitive, rapid and cost efficient method that can detect active botulinum neurotoxin (BoNT) in complex biological samples such as foods or serum is desired in order to 1) counter the potential bioterrorist threat 2) enhance food safety 3) enable future pharmacokinetic studies in medical applications that utilize BoNTs.
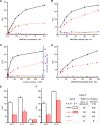
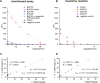
Methodology/principal findings: Here we describe a botulinum neurotoxin serotype A assay with a large immuno-sorbent surface area (BoNT/A ALISSA) that captures a low number of toxin molecules and measures their intrinsic metalloprotease activity with a fluorogenic substrate. In direct comparison with the "gold standard" mouse bioassay, the ALISSA is four to five orders of magnitudes more sensitive and considerably faster. Our method reaches attomolar sensitivities in serum, milk, carrot juice, and in the diluent fluid used in the mouse assay. ALISSA has high specificity for the targeted type A toxin when tested against alternative proteases including other BoNT serotypes and trypsin, and it detects the holotoxin as well as the multi-protein complex form of BoNT/A. The assay was optimized for temperature, substrate concentration, size and volume proportions of the immuno-sorbent matrix, enrichment and reaction times. Finally, a kinetic model is presented that is consistent with the observed improvement in sensitivity.
Conclusions/significance: The sensitivity, specificity, speed and simplicity of the BoNT ALISSA should make this method attractive for diagnostic, biodefense and pharmacological applications.
Conflict of interest statement
Figures

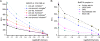
References
-
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, et al. Botulinum toxin as a biological weapon: medical and public health management. Jama. 2001;285:1059–1070. - PubMed
-
- Long SS. Infant botulism and treatment with BIG-IV (BabyBIG®). Pediatr Infect Dis J. 2007;26:261–262. - PubMed
-
- Koepke R, Sobel J, Arnon, S S. Global occurrence of infant botulism, 1976–2006. Pediatrics. 2008 [in press] - PubMed
-
- Arnon SS, Schechter R, Maslanka SE, Jewell NP, Hatheway CL. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med. 2006;354:462–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

