Pancreatic insulin content regulation by the estrogen receptor ER alpha
- PMID: 18446233
- PMCID: PMC2323613
- DOI: 10.1371/journal.pone.0002069
Pancreatic insulin content regulation by the estrogen receptor ER alpha
Abstract
The function of pancreatic beta-cells is the synthesis and release of insulin, the main hormone involved in blood glucose homeostasis. Estrogen receptors, ER alpha and ER beta, are important molecules involved in glucose metabolism, yet their role in pancreatic beta-cell physiology is still greatly unknown. In this report we show that both ER alpha and ER beta are present in pancreatic beta-cells. Long term exposure to physiological concentrations of 17beta-estradiol (E2) increased beta-cell insulin content, insulin gene expression and insulin release, yet pancreatic beta-cell mass was unaltered. The up-regulation of pancreatic beta-cell insulin content was imitated by environmentally relevant doses of the widespread endocrine disruptor Bisphenol-A (BPA). The use of ER alpha and ER beta agonists as well as ER alphaKO and ER betaKO mice suggests that the estrogen receptor involved is ER alpha. The up-regulation of pancreatic insulin content by ER alpha activation involves ERK1/2. These data may be important to explain the actions of E2 and environmental estrogens in endocrine pancreatic function and blood glucose homeostasis.
Conflict of interest statement
Figures
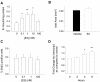
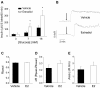
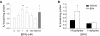
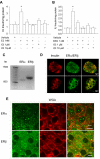


References
-
- Ropero AB, Alonso-Magdalena P, Quesada I, Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2007:doi:10.1016/j.steroids.2007.12.018 [Online 27 December 2007]. - PubMed
-
- Rochira V, Balestrieri A, Madeo B, Spaggiari A, Carani C. Congenital estrogen deficiency in men: a new syndrome with different phenotypes; clinical and therapeutic implications in men. Mol Cell Endocrinol. 2002;193:19–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

