Rescue of salivary gland function after stem cell transplantation in irradiated glands
- PMID: 18446241
- PMCID: PMC2329592
- DOI: 10.1371/journal.pone.0002063
Rescue of salivary gland function after stem cell transplantation in irradiated glands
Abstract
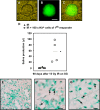
Head and neck cancer is the fifth most common malignancy and accounts for 3% of all new cancer cases each year. Despite relatively high survival rates, the quality of life of these patients is severely compromised because of radiation-induced impairment of salivary gland function and consequential xerostomia (dry mouth syndrome). In this study, a clinically applicable method for the restoration of radiation-impaired salivary gland function using salivary gland stem cell transplantation was developed. Salivary gland cells were isolated from murine submandibular glands and cultured in vitro as salispheres, which contained cells expressing the stem cell markers Sca-1, c-Kit and Musashi-1. In vitro, the cells differentiated into salivary gland duct cells and mucin and amylase producing acinar cells. Stem cell enrichment was performed by flow cytrometric selection using c-Kit as a marker. In vitro, the cells differentiated into amylase producing acinar cells. In vivo, intra-glandular transplantation of a small number of c-Kit(+) cells resulted in long-term restoration of salivary gland morphology and function. Moreover, donor-derived stem cells could be isolated from primary recipients, cultured as secondary spheres and after re-transplantation ameliorate radiation damage. Our approach is the first proof for the potential use of stem cell transplantation to functionally rescue salivary gland deficiency.
Conflict of interest statement
Figures






References
-
- Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:213–225. - PubMed
-
- Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. - PubMed
-
- Gresik EW. The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microsc Res Tech. 1994;27:1–24. - PubMed
-
- Denny PC, Ball WD, Redman RS. Salivary glands: a paradigm for diversity of gland development. Crit Rev Oral Biol Med. 1997;8:51–75. - PubMed
-
- Burford-Mason AP, Cummins MM, Brown DH, MacKay AJ, Dardick I. Immunohistochemical analysis of the proliferative capacity of duct and acinar cells during ligation-induced atrophy and subsequent regeneration of rat parotid gland. J Oral Pathol Med. 1993;22:440–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

