Cataleptic effects of gamma-hydroxybutyrate (GHB) and baclofen in mice: mediation by GABA(B) receptors, but differential enhancement by N-methyl-d-aspartate (NMDA) receptor antagonists
- PMID: 18446324
- PMCID: PMC3470870
- DOI: 10.1007/s00213-008-1160-5
Cataleptic effects of gamma-hydroxybutyrate (GHB) and baclofen in mice: mediation by GABA(B) receptors, but differential enhancement by N-methyl-d-aspartate (NMDA) receptor antagonists
Abstract
Rationale: Gamma-hydroxybutyrate (GHB) is a gamma-aminobutyric acid (GABA) analog that is used to treat narcolepsy but that is also abused. GHB has many actions in common with the GABA(B) receptor agonist baclofen, but their underlying GABA(B) receptor mechanisms may be different.
Objective: The aim of this study is to further investigate a possible differential role of glutamate in GABA(B) receptor-mediated effects of GHB and baclofen.
Materials and methods: The experiments examined the effects of non-competitive antagonists at the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors on GHB-induced catalepsy and compared these effects with those on baclofen-induced catalepsy.
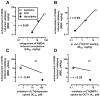
Results: In C57BL/6J mice, ketamine, phencyclidine (PCP), and dizocilpine (MK-801) all enhanced GHB-induced catalepsy. They did so with a potency order (i.e., MK-801 > PCP > ketamine) consistent with their relative potencies as NMDA antagonists but not as inhibitors of dopamine or organic cation transporters. Ketamine, PCP, and MK-801 enhanced catalepsy along inverted U-shaped dose-response curves likely because higher doses affected motor coordination, which limited their catalepsy-enhancing effects. Doses that were maximally effective to enhance GHB-induced catalepsy did not affect the cataleptic effects of baclofen.
Conclusions: The finding that NMDA receptor antagonists enhance the cataleptic effects of GHB but not those of baclofen is further evidence that the GABA(B) receptor mechanisms mediating the effects of GHB and GABA(B) agonists are not identical. Differential interactions of glutamate with the GABA(B) receptor mechanisms mediating the effects of GHB and baclofen may explain why GHB is effective for treating narcolepsy and is abused, whereas baclofen is not.
Figures

Similar articles
-
Cataleptic effects of gamma-hydroxybutyrate (GHB), its precursor gamma-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348.Psychopharmacology (Berl). 2007 Jun;192(3):407-14. doi: 10.1007/s00213-007-0718-y. Epub 2007 Feb 3. Psychopharmacology (Berl). 2007. PMID: 17277933
-
Synergistic interactions between 'club drugs': gamma-hydroxybutyrate and phencyclidine enhance each other's discriminative stimulus effects.Behav Pharmacol. 2007 Dec;18(8):807-10. doi: 10.1097/FBP.0b013e3282f18d45. Behav Pharmacol. 2007. PMID: 17989519
-
Involvement of GABA(A) and GABA(B) receptors in the mediation of discriminative stimulus effects of gamma-hydroxybutyric acid.Physiol Behav. 1998 Jun 1;64(3):293-302. doi: 10.1016/s0031-9384(98)00062-6. Physiol Behav. 1998. PMID: 9748096
-
Behavioral analyses of GHB: receptor mechanisms.Pharmacol Ther. 2009 Jan;121(1):100-14. doi: 10.1016/j.pharmthera.2008.10.003. Epub 2008 Oct 29. Pharmacol Ther. 2009. PMID: 19010351 Free PMC article. Review.
-
Brain NMDA Receptors in Schizophrenia and Depression.Biomolecules. 2020 Jun 23;10(6):947. doi: 10.3390/biom10060947. Biomolecules. 2020. PMID: 32585886 Free PMC article. Review.
Cited by
-
Metabolomic study of polyamines in rat urine following intraperitoneal injection of γ-hydroxybutyric acid.Metabolomics. 2019 Apr 2;15(4):58. doi: 10.1007/s11306-019-1517-2. Metabolomics. 2019. PMID: 30941522
-
Differential effects of GABAB receptor subtypes, {gamma}-hydroxybutyric Acid, and Baclofen on EEG activity and sleep regulation.J Neurosci. 2010 Oct 20;30(42):14194-204. doi: 10.1523/JNEUROSCI.3145-10.2010. J Neurosci. 2010. PMID: 20962240 Free PMC article.
-
GABAB receptor-positive modulators: enhancement of GABAB receptor agonist effects in vivo.J Pharmacol Exp Ther. 2010 Oct;335(1):163-71. doi: 10.1124/jpet.110.171116. Epub 2010 Jul 13. J Pharmacol Exp Ther. 2010. PMID: 20628000 Free PMC article.
-
GHB pharmacology and toxicology: acute intoxication, concentrations in blood and urine in forensic cases and treatment of the withdrawal syndrome.Curr Neuropharmacol. 2015 Jan;13(1):47-70. doi: 10.2174/1570159X13666141210215423. Curr Neuropharmacol. 2015. PMID: 26074743 Free PMC article. Review.
-
Behavioral effects of gamma-hydroxybutyrate, its precursor gamma-butyrolactone, and GABA(B) receptor agonists: time course and differential antagonism by the GABA(B) receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348).J Pharmacol Exp Ther. 2009 Sep;330(3):876-83. doi: 10.1124/jpet.109.151845. Epub 2009 Jun 29. J Pharmacol Exp Ther. 2009. PMID: 19564487 Free PMC article.
References
-
- Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. - PubMed
-
- Benavides J, Rumigny JF, Bourguignon JJ, Cash C, Wermuth CG, Mandel P, Vincendon G, Maitre M. High affinity binding sites for gamma-hydroxybutyric acid in rat brain. Life Sci. 1982;30:953–961. - PubMed
-
- Bonanno G, Raiteri M. Functional evidence for multiple gamma-aminobutyric acid B receptor subtypes in the rat cerebral cortex. J Pharmacol Exp Ther. 1992;262:114–118. - PubMed
-
- Carai MA, Colombo G, Brunetti G, Melis S, Serra S, Vacca G, Mastinu S, Pistuddi AM, Solinas C, Cignarella G, Minardi G, Gessa GL. Role of GABA(B) receptors in the sedative/hypnotic effect of gamma-hydroxybutyric acid. Eur J Pharmacol. 2001;428:315–321. - PubMed
-
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABAB receptors in the discriminative stimulus effects of gamma-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

