Orthopaedic surgeons prefer to participate in expertise-based randomized trials
- PMID: 18446421
- PMCID: PMC2505251
- DOI: 10.1007/s11999-008-0273-9
Orthopaedic surgeons prefer to participate in expertise-based randomized trials
Abstract
Empiric data and theoretical arguments suggest an alternative randomized clinical trial (RCT) design, called expertise-based RCT, has enhanced validity, applicability, and ethical integrity compared with conventional RCT. Little is known, however, about whether physicians will participate in an expertise-based RCT. In a cross-sectional survey of Canadian orthopaedic surgeons, we evaluated preference for and willingness to participate in an expertise-based versus a conventional RCT if given the opportunity to participate in a trial investigating the effectiveness of high tibial osteotomy versus unicompartmental knee arthroplasty. Using an electronic survey ((c)2005 SurveyMonkey.com), we invited all 767 members of the Canadian Orthopaedic Association (2005) to participate; 276 surgeons completed the questionnaire (37.5% response rate). One hundred two surgeons (53.4%) were willing to participate in an expertise-based RCT compared with 35 surgeons (18.3%) willing to participate in a conventional RCT. Ninety-seven surgeons (52.4%) strongly or moderately preferred the expertise-based design compared with 25 (13.5%) who preferred the conventional design. For the clinical example we presented, the majority of Canadian orthopaedic surgeons were willing to participate in and preferred the expertise-based design. The expertise-based randomized clinical trial design may overcome some of the barriers to conducting clinical trials in orthopaedic surgery and improve the validity of their conclusions.
Figures

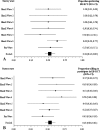
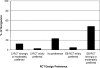

Comment in
-
Letter to the editor re: Orthopaedic surgeons prefer to participate in expertise-based randomized trials: Bednarska E, Bryant D, Devereaux, PJ. Orthopaedic surgeons prefer to participate in expertise-based randomized trials. Clin Orthop Relat Res. 2008;466:1734-1744.Clin Orthop Relat Res. 2009 Jan;467(1):298-300; author reply 301-2. doi: 10.1007/s11999-008-0575-y. Epub 2008 Oct 24. Clin Orthop Relat Res. 2009. PMID: 18949527 Free PMC article. No abstract available.
Similar articles
-
Letter to the editor re: Orthopaedic surgeons prefer to participate in expertise-based randomized trials: Bednarska E, Bryant D, Devereaux, PJ. Orthopaedic surgeons prefer to participate in expertise-based randomized trials. Clin Orthop Relat Res. 2008;466:1734-1744.Clin Orthop Relat Res. 2009 Jan;467(1):298-300; author reply 301-2. doi: 10.1007/s11999-008-0575-y. Epub 2008 Oct 24. Clin Orthop Relat Res. 2009. PMID: 18949527 Free PMC article. No abstract available.
-
Open versus endovascular repair of abdominal aortic aneurysm: a survey of Canadian vascular surgeons.Can J Surg. 2008 Apr;51(2):142-8; quiz 149. Can J Surg. 2008. PMID: 18377756 Free PMC article.
-
Willingness to participate in placebo-controlled surgical trials of the knee.Bone Joint J. 2024 Dec 1;106-B(12):1408-1415. doi: 10.1302/0301-620X.106B12.BJJ-2023-1266.R2. Bone Joint J. 2024. PMID: 39615522
-
Unicompartmental Knee Arthroplasty vs High Tibial Osteotomy for Knee Osteoarthritis: A Systematic Review and Meta-Analysis.J Arthroplasty. 2018 Mar;33(3):952-959. doi: 10.1016/j.arth.2017.10.025. Epub 2017 Dec 2. J Arthroplasty. 2018. PMID: 29203354
-
[Knee osteotomy versus unicompartmental knee replacement in the treatment of unicompartmental knee arthritis].Rev Med Chir Soc Med Nat Iasi. 2006 Jul-Sep;110(3):582-9. Rev Med Chir Soc Med Nat Iasi. 2006. PMID: 17571549 Review. Romanian.
Cited by
-
How to design high quality acupuncture trials-a consensus informed by evidence.BMJ. 2022 Mar 30;376:e067476. doi: 10.1136/bmj-2021-067476. BMJ. 2022. PMID: 35354583 Free PMC article.
-
Methodological choices for the clinical development of medical devices.Med Devices (Auckl). 2014 Sep 23;7:325-34. doi: 10.2147/MDER.S63869. eCollection 2014. Med Devices (Auckl). 2014. PMID: 25285025 Free PMC article. Review.
-
A systematic review of the use of an expertise-based randomised controlled trial design.Trials. 2015 May 30;16:241. doi: 10.1186/s13063-015-0739-5. Trials. 2015. PMID: 26025450 Free PMC article.
-
Characterization of medical device randomized controlled trials with adaptive designs.J Comp Eff Res. 2025 Jan;14(1):e240011. doi: 10.57264/cer-2024-0011. Epub 2024 Dec 9. J Comp Eff Res. 2025. PMID: 39656083 Free PMC article.
-
Design and execution of clinical trials in orthopaedic surgery.Bone Joint Res. 2014 May;3(5):161-8. doi: 10.1302/2046-3758.35.2000280. Bone Joint Res. 2014. PMID: 24869465 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1056/NEJMsa011788', 'is_inner': False, 'url': 'https://doi.org/10.1056/nejmsa011788'}, {'type': 'PubMed', 'value': '11948274', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11948274/'}]}
- Begg CB, Riedel ER, Bach PB, Kattan MW, Schrag D, Warren JL, Scardino PT. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–1144. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1056/NEJMsa035205', 'is_inner': False, 'url': 'https://doi.org/10.1056/nejmsa035205'}, {'type': 'PubMed', 'value': '14645640', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/14645640/'}]}
- Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1136/bmj.38173.577697.55', 'is_inner': False, 'url': 'https://doi.org/10.1136/bmj.38173.577697.55'}, {'type': 'PMC', 'value': 'PMC514200', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC514200/'}, {'type': 'PubMed', 'value': '15298881', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15298881/'}]}
- Bridgewater B, Grayson AD, Au J, Hassan R, Dihmis WC, Munsch C, Waterworth P. Improving mortality of coronary surgery over first four years of independent practice: retrospective examination of prospectively collected data from 15 surgeons. BMJ. 2004;329:421. - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1136/bmj.330.7482.88', 'is_inner': False, 'url': 'https://doi.org/10.1136/bmj.330.7482.88'}, {'type': 'PMC', 'value': 'PMC543877', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC543877/'}, {'type': 'PubMed', 'value': '15637373', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15637373/'}]}
- Devereaux PJ, Bhandari M, Clarke M, Montori VM, Cook DJ, Yusuf S, Sackett DL, Cina CS, Walter SD, Haynes B, Schunemann HJ, Norman GR, Guyatt GH. Need for expertise based randomised controlled trials. BMJ. 2005;330:88. - PMC - PubMed
-
- None
- Dillman DA. Mail and Telephone Surveys: The Total Design Method. Toronto, Canada: John Wiley & Sons; 1978.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

