Design and evaluation of microemulsions for improved parenteral delivery of propofol
- PMID: 18446474
- PMCID: PMC2976909
- DOI: 10.1208/s12249-007-9023-7
Design and evaluation of microemulsions for improved parenteral delivery of propofol
Abstract
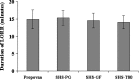
The objective of this investigation was to evaluate the potential of the microemulsions to improve the parenteral delivery of propofol. Pseudo-ternary phase diagrams were plotted to identify microemulsification region of propofol. The propofol microemulsions were evaluated for globule size, physical and chemical stability, osmolarity, in vitro hemolysis, pain caused by injection using rat paw-lick test and in vivo anesthetic activity. The microemulsions exhibited globule size less than 25 nm and demonstrated good physical and chemical stability. Propofol microemulsions were slightly hypertonic and resulted in less than 1% hemolysis after 2 h of storage with human blood at 37 degrees C. Rat paw-lick test indicated that propofol microemulsions were significantly less painful as compared to the marketed propofol formulation. The anesthetic activity of the microemulsions was similar to the marketed propofol formulation indicating that they do not compromise the pharmacological action of propofol. The stability studies indicated that the microemulsions were stable for 3 months when stored at 5 +/- 3 degrees C. Thus, microemulsions appeared to be an interesting alternative to the current propofol formulations.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

