Development of lapachol topical formulation: anti-inflammatory study of a selected formulation
- PMID: 18446477
- PMCID: PMC2976889
- DOI: 10.1208/s12249-007-9002-z
Development of lapachol topical formulation: anti-inflammatory study of a selected formulation
Abstract
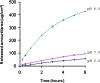
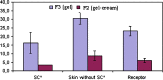
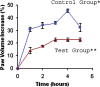
This study aimed at developing a topical formulation of lapachol, a compound isolated from various Bignoniaceae species and at evaluating its topical anti-inflammatory activity. The influence of the pharmaceutical form and different types of emulsifiers was evaluated by in-vitro release studies. The formulations showing the highest release rate were selected and assessed trough skin permeation and retention experiments. It was observed that the gel formulation provided significantly higher permeation and retained amount (3.9-fold) of lapachol as compared to the gel-cream formulation. Antinociceptive and antiedematogenic activities of the most promising formulation were also evaluated. Lapachol gel reduced the increase in hind-paw volume induced by carrageenan injection and reduced nociception produced by acetic acid (0.8% in water, i.p.) when used topically. These results suggest that topical delivery of lapachol from gel formulations may be an effective medication for both dermal and subdermal injuries.
Figures


Similar articles
-
Microemulsion Formulations for the Transdermal Delivery of Lapachol.AAPS PharmSciTech. 2018 May;19(4):1837-1846. doi: 10.1208/s12249-018-0995-2. Epub 2018 Apr 10. AAPS PharmSciTech. 2018. PMID: 29637497
-
Development of an effective topical liposomal formulation for localized analgesia and anti-inflammatory actions in the Complete Freund's Adjuvant rodent model of acute inflammatory pain.Pain Physician. 2014 Nov-Dec;17(6):E719-35. Pain Physician. 2014. PMID: 25415787 Clinical Trial.
-
Formulation and evaluation of curcumin gel for topical application.Pharm Dev Technol. 2009;14(1):80-9. doi: 10.1080/10837450802409438. Pharm Dev Technol. 2009. PMID: 18821270
-
Emerging Technologies to Target Drug Delivery to the Skin - the Role of Crystals and Carrier-Based Systems in the Case Study of Dapsone.Pharm Res. 2020 Nov 9;37(12):240. doi: 10.1007/s11095-020-02951-4. Pharm Res. 2020. PMID: 33169237 Review.
-
Advances and challenges in serratiopeptidase topical formulation.Ann Pharm Fr. 2024 Nov;82(6):966-979. doi: 10.1016/j.pharma.2024.05.008. Epub 2024 May 29. Ann Pharm Fr. 2024. PMID: 38821483 Review.
Cited by
-
Safety and tolerability of Pau d' Arco (Tabebuia avellanedae) for primary dysmenorrhea: A single-arm, open-label trial on adults ages 18-45.Adv Integr Med. 2022 Sep;9(3):159-166. doi: 10.1016/j.aimed.2022.04.003. Epub 2022 May 7. Adv Integr Med. 2022. PMID: 36960315 Free PMC article.
-
Enhanced antitumor efficacy of lapachol-loaded nanoemulsion in breast cancer tumor model.Biomed Pharmacother. 2021 Jan;133:110936. doi: 10.1016/j.biopha.2020.110936. Epub 2020 Nov 27. Biomed Pharmacother. 2021. PMID: 33254016 Free PMC article.
-
In Vitro Evaluation of Novel Hybrid Cooperative Complexes in a Wound Healing Model: A Step Toward Improved Bioreparation.Int J Mol Sci. 2019 Sep 24;20(19):4727. doi: 10.3390/ijms20194727. Int J Mol Sci. 2019. PMID: 31554177 Free PMC article.
-
Nanoemulgel, an Innovative Carrier for Diflunisal Topical Delivery with Profound Anti-Inflammatory Effect: in vitro and in vivo Evaluation.Int J Nanomedicine. 2021 Feb 22;16:1457-1472. doi: 10.2147/IJN.S294653. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33654396 Free PMC article.
-
Determination of Photosensitizing Potential of Lapachol for Photodynamic Inactivation of Bacteria.Molecules. 2024 Nov 2;29(21):5184. doi: 10.3390/molecules29215184. Molecules. 2024. PMID: 39519826 Free PMC article.
References
-
- Araújo E. L., Alencar J. R. B., Rolim Neto P. J. Lapachol: segurança e eficácia na terapêutica. Rev. Bras. Farmacogn. 2002;12:57–59. doi: 10.1590/S0102-695X2002000300028. - DOI
-
- Fonseca S. G. C., Braga R. M. C., Santana D. P. Lapachol. chemistry, pharmacology and assay methods. Rev. Bras. Farm. 2003;84:9–16.
-
- Lui C. Y., Ayeni A. A., Gyllenhaal C., Groves M. J. Some formulation properties of Lapachol, A potential oncolytic agent of natural origin. Drug. Dev. Ind. Pharm. 1985;11:63–79. doi: 10.3109/03639048509057698. - DOI
-
- Wanick M. C., Bandeira J. A., Fernandes R. V. Ação antiinflamatória e cicatrizante do extrato hidroalcoólico do Líber do Pau d’arco Roxo (Tabebuia avellanedae), em pacientes portadores de Cervicites e Cérvico-vaginites. Revista do instituto de Antibióticos. 1970;10:41–46.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

