Cell cycle checkpoint models for cellular pharmacology of paclitaxel and platinum drugs
- PMID: 18446502
- PMCID: PMC2751448
- DOI: 10.1208/s12248-007-9003-6
Cell cycle checkpoint models for cellular pharmacology of paclitaxel and platinum drugs
Abstract
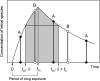
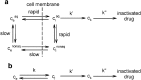
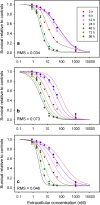
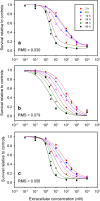
A pharmacokinetic-pharmacodynamic mathematical model is developed for cellular pharmacology of chemotherapeutic drugs for which the decisive step towards cell death occurs at a point in the cell cycle, presumably corresponding to a cell cycle checkpoint. For each cell, the model assumes a threshold level of some intracellular species at that checkpoint, beyond which the cell dies. The threshold level is assumed to have a log-normal distribution in the cell population. The kinetics of formation of the lethal intracellular species depends on the drug, and on the cellular pharmacokinetics and binding kinetics of the cell. Specific models are developed for paclitaxel and for platinum drugs (cisplatin, oxaliplatin and carboplatin). In the case of paclitaxel, two separate mechanisms of cell death necessitate a model that accounts for two checkpoints, with different intracellular species. The model was tested on a number of in vitro cytotoxicity data sets for these drugs, and found overall to give significantly better fits than previously proposed cellular pharmacodynamic models. It provides an explanation for the asymptotic convergence of dose-response curves as exposure time becomes long.
Figures
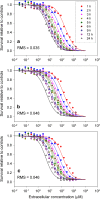
Similar articles
-
Cell cycle checkpoint efficiency and cellular response to paclitaxel in prostate cancer cells.Prostate. 2001 Sep 15;48(4):254-64. doi: 10.1002/pros.1105. Prostate. 2001. PMID: 11536305
-
Chk1 Inhibitor SCH900776 Effectively Potentiates the Cytotoxic Effects of Platinum-Based Chemotherapeutic Drugs in Human Colon Cancer Cells.Neoplasia. 2017 Oct;19(10):830-841. doi: 10.1016/j.neo.2017.08.002. Epub 2017 Sep 6. Neoplasia. 2017. PMID: 28888100 Free PMC article.
-
Analysis of cell-cycle checkpoint pathways in head and neck cancer cell lines: implications for therapeutic strategies.Arch Otolaryngol Head Neck Surg. 2002 Feb;128(2):167-76. doi: 10.1001/archotol.128.2.167. Arch Otolaryngol Head Neck Surg. 2002. PMID: 11843726
-
The integration of paclitaxel and new platinum compounds in the treatment of advanced ovarian cancer.Int J Gynecol Cancer. 2001;11 Suppl 1:21-30. doi: 10.1046/j.1525-1438.2001.11(suppl.1)sup1021.x. Int J Gynecol Cancer. 2001. PMID: 11488999 Review.
-
Membrane transporters as determinants of the pharmacology of platinum anticancer drugs.Curr Cancer Drug Targets. 2012 Oct;12(8):962-86. doi: 10.2174/156800912803251199. Curr Cancer Drug Targets. 2012. PMID: 22794121 Review.
Cited by
-
Towards an integrated systems-based modelling framework for drug transport and its effect on tumour cells.J Biol Eng. 2014 Jan 13;8:3. doi: 10.1186/1754-1611-8-3. eCollection 2014. J Biol Eng. 2014. PMID: 24764492 Free PMC article.
-
The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin.Sci Rep. 2019 Apr 4;9(1):5627. doi: 10.1038/s41598-019-41903-w. Sci Rep. 2019. PMID: 30948733 Free PMC article.
-
The relationships between the chemosensitivity of human gastric cancer to paclitaxel and the expressions of class III β-tubulin, MAPT, and survivin.Med Oncol. 2014 May;31(5):950. doi: 10.1007/s12032-014-0950-3. Epub 2014 Apr 11. Med Oncol. 2014. PMID: 24722794
-
Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues.Front Oncol. 2013 Nov 18;3:278. doi: 10.3389/fonc.2013.00278. Front Oncol. 2013. PMID: 24303366 Free PMC article. Review.
-
The additive damage model: a mathematical model for cellular responses to drug combinations.J Theor Biol. 2014 Sep 21;357:10-20. doi: 10.1016/j.jtbi.2014.04.032. Epub 2014 May 4. J Theor Biol. 2014. PMID: 24799130 Free PMC article.
References
-
- Briasoulis E., Karavasilis V., Tzamakou E., Haidou C., Piperidou C., Pavlidis N. Pharmacodynamics of non-break weekly paclitaxel (Taxol) and pharmacokinetics of Cremophor-EL vehicle: results of a dose-escalation study. Anticancer Drugs. 2002;13(5):481–489. doi: 10.1097/00001813-200206000-00006. - DOI - PubMed
-
- El-Kareh A. W., Secomb T. W. Theoretical analyses and simulations of anticancer drug delivery. In: Brown D. M., editor. Drug Delivery Systems in Cancer Therapy. Clifton, NJ: Humana Press; 2003. pp. 25–41.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

