Pharmacokinetically-guided lead optimization of nitrofuranylamide anti-tuberculosis agents
- PMID: 18446516
- PMCID: PMC2751462
- DOI: 10.1208/s12248-008-9017-8
Pharmacokinetically-guided lead optimization of nitrofuranylamide anti-tuberculosis agents
Abstract
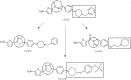
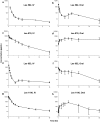
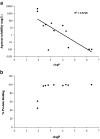
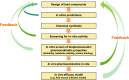
In an effort to develop novel and more potent therapies to treat tuberculosis, a new class of chemical agents, nitrofuranylamides, is being developed. The present study examines biopharmaceutic properties and preclinical pharmacokinetics of nitrofuranylamides at early stages of drug discovery to accelerate the optimization of leads into development candidates. The first tested compound, Lee 562, had high anti-tuberculosis activity in vitro, but exhibited poor metabolic stability resulting in a high systemic clearance, a short elimination half-life and low oral bioavailability in vivo in rats. Thus, two follow-up compounds were designed and tested that included structural modifications for increased metabolic stability. Both compounds showed improved metabolic stability compared to Lee 562, with Lee 878 being much more stable than Lee 952. As a consequence, the oral bioavailability of Lee 878 reached approximately 27% compared to 16% for the other two compounds. This observation prompted us to select compounds based on metabolic stability screening and a new set of nine compounds with high in vitro activity were tested for metabolic stability. The most stable compound in the assay, Lee 1106 was selected for further pharmacokinetic evaluation in rats. Surprisingly, Lee 1106 exhibited poor oral bioavailability, 4.6%. Biopharmaceutic evaluation of the compound showed that the compound has poor aqueous solubility and a high clogP. Based on these results, a screening paradigm was developed for optimization of the nitrofuranylamide lead compounds in a timely and cost-effective manner that might also be applicable to other classes of anti-infective drugs.
Figures

Similar articles
-
Antitubercular nitrofuran isoxazolines with improved pharmacokinetic properties.Bioorg Med Chem. 2012 Oct 15;20(20):6063-72. doi: 10.1016/j.bmc.2012.08.023. Epub 2012 Aug 28. Bioorg Med Chem. 2012. PMID: 22995771 Free PMC article.
-
Pentacyclic nitrofurans with in vivo efficacy and activity against nonreplicating Mycobacterium tuberculosis.PLoS One. 2014 Feb 5;9(2):e87909. doi: 10.1371/journal.pone.0087909. eCollection 2014. PLoS One. 2014. PMID: 24505329 Free PMC article.
-
Nitrofurans as novel anti-tuberculosis agents: identification, development and evaluation.Curr Top Med Chem. 2007;7(5):509-26. doi: 10.2174/156802607780059772. Curr Top Med Chem. 2007. PMID: 17346196 Review.
-
Synthesis and evaluation of nitrofuranylamides as novel antituberculosis agents.J Med Chem. 2004 Oct 7;47(21):5276-83. doi: 10.1021/jm049972y. J Med Chem. 2004. PMID: 15456272
-
Preclinical pharmacokinetics: an approach towards safer and efficacious drugs.Curr Drug Metab. 2006 Feb;7(2):165-82. doi: 10.2174/138920006775541552. Curr Drug Metab. 2006. PMID: 16472106 Review.
Cited by
-
Aminomethyl spectinomycins as therapeutics for drug-resistant respiratory tract and sexually transmitted bacterial infections.Sci Transl Med. 2015 May 20;7(288):288ra75. doi: 10.1126/scitranslmed.3010572. Sci Transl Med. 2015. PMID: 25995221 Free PMC article.
-
Spectinamides: a new class of semisynthetic antituberculosis agents that overcome native drug efflux.Nat Med. 2014 Feb;20(2):152-158. doi: 10.1038/nm.3458. Epub 2014 Jan 26. Nat Med. 2014. PMID: 24464186 Free PMC article.
-
Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections.Nat Rev Microbiol. 2011 Jan;9(1):62-75. doi: 10.1038/nrmicro2474. Nat Rev Microbiol. 2011. PMID: 21164535 Free PMC article. Review.
-
A microbiological assessment of novel nitrofuranylamides as anti-tuberculosis agents.J Antimicrob Chemother. 2008 Nov;62(5):1037-45. doi: 10.1093/jac/dkn307. Epub 2008 Aug 7. J Antimicrob Chemother. 2008. PMID: 18693235 Free PMC article.
-
Noscapinoids with anti-cancer activity against human acute lymphoblastic leukemia cells (CEM): a three dimensional chemical space pharmacophore modeling and electronic feature analysis.J Mol Model. 2012 Jan;18(1):307-18. doi: 10.1007/s00894-011-1057-9. Epub 2011 Apr 27. J Mol Model. 2012. PMID: 21523542
References
-
- Global Alliance for TB Drug Development. www.tballiance.org (2007).
-
- World Health Organization. Fact sheet on Tuberculosis. Geneva: WHO (March, 2006).
-
- Center for Disease Control and Prevention. Morbidity and mortality weekly report. Atlanta: CDC (Apr 2006).
-
- Burman W. J., Jones B. E. Treatment of HIV-related tuberculosis in the era of effective antiretroviral therapy. Am. J. Respir. Crit. Care Med. 2001;164(1):7–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

