Use of the Biopharmaceutical Classification System in early drug development
- PMID: 18446521
- PMCID: PMC2751465
- DOI: 10.1208/s12248-008-9020-0
Use of the Biopharmaceutical Classification System in early drug development
Abstract
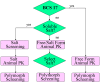
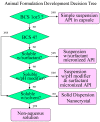
The Biopharmaceutics Classification System (BCS) is not only a useful tool for obtaining waivers for in-vivo bioequivalence studies but also for decision making in the discovery and early development of new drugs. Measurement of solubility and permeability in the discovery/development settings is described. These data can be utilized for the preliminary BCS classification of pipeline compounds. A decision tree is described in the prioritization of salt and polymorph screening studies prior to in vivo studies in animals. For BCS class 1 and 3 compounds, polymorphism is less likely to impact on bioavailability. The polymorph screening study may be postponed after animal studies. The BCS classification can also be used in the design of animal and human formulations. A BCS-based animal formulation development decision tree is presented. A compound is triaged based on a series of decision points into one of the five formulation strategies. Human formulation has different requirements than animal formulation. A comparison between animal and human formulation strategies is presented. In conclusion, for non-BCS 1 compounds, the right-first-time polymorph and formulation selection ensures consistent pharmacokinetic performance and avoids bridging BA/BE studies. It is in line with FDA's initiative to reduce R&D cycle time through quality by design for pharmaceutical products.
Figures
References
-
- Food and Drug Administration. Guidance for industry, waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system, Food and Drug Administration, Rockville, MD, 2000. Available at http://www.fda.gov/cder/guidance/index.htm.
-
- World Health Organization. Proposal to waive in vivo bioequivalence requirements for WHO model list of essential medicines immediate-release, solid oral dosage forms. World Health Organization, Technical Report Series no. 937 (2006). Available at http://www.who.int/prequal/info_general/documents/TRS937/WHO_TRS_937__an...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical