Targeting TARP, a novel breast and prostate tumor-associated antigen, with T cell receptor-like human recombinant antibodies
- PMID: 18446790
- PMCID: PMC2682370
- DOI: 10.1002/eji.200737524
Targeting TARP, a novel breast and prostate tumor-associated antigen, with T cell receptor-like human recombinant antibodies
Abstract
MHC class I molecules are important components of immune surveillance. There are no available methods to directly visualize and determine the quantity and distribution of MHC/peptide complexes on individual cells or to detect such complexes on antigen-presenting cells in tissues. MHC-restricted recombinant antibodies with the same specificity of T cell receptors (TCR) may become a valuable tool to address these questions. They may also serve as valuable targeting molecules that mimic the specificity of cytotoxic T cells. We isolated by phage display a panel of human recombinant Fab antibodies with peptide-specific, MHC-restricted TCR-like reactivity directed toward HLA-A2-restricted T cell epitopes derived from a novel antigen termed TCRgamma alternative reading frame protein (TARP) which is expressed on prostate and breast cancer cells. We have characterized one of these recombinant antibodies and demonstrated its capacity to directly detect specific HLA-A2/TARP T cell epitopes on antigen-presenting cells that have complexes formed by naturally occurring active intracellular processing of the antigen, as well as on the surface of tumor cells. Moreover, by genetic fusion we armed the TCR-like antibody with a potent toxin and demonstrated that it can serve as a targeting moiety killing tumor cells in a peptide-specific, MHC-restricted manner similar to cytotoxic T lymphocytes.
Figures
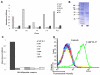
ELISA reactivity assay of Fab antibody clones with recombinant purified TARP/HLA-A2 and control complexes. Biotinylated scHLA-A2-peptide complexes were immobilized through BSA-streptavidin. Detection of Fab binding was with Peroxidase-labeld anti-human Fab.
Expression and purification of TCR-like D2 Fab. SDS-PAGE analysis of purified D2 Fab. Fab clone was expressed in E.coli and purified by metal affinity chromatography as described. M: Molecular weight markers, NR: Non-reduced SDS-PAGE.
ELISA assay for D2 Fab reactivity with TRAP/HLA-A2 and control recombinant purified complexes. Detection was with Peroxidase-labeled anti-human Fab.
Binding of D2 Fab by flow cytometry to peptide-loaded APCs. JY EBV-transformed HLA-A2 positive B cells were loaded with the TARP29-37 and control HLA-A2-restricted peptides and the reactivity with purified D2 Fab (10-50μg/ml) was tested. Detection of binding was with PE-labeled anti-human Fab. Control peptides were: MART1, hTERT (540), gp 100-derived peptides G9-209, G9-280, Human T cell lymphotropic virus type 1(HTLV-1) derived peptide TAX and Cytomegalovirus (CMV)- derived peptide. Data are representative of 5 experiments.
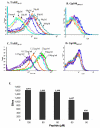

ELISA binding assay of D2 Fab to recombinant purified HLA-A2 complexes generated with wild-type and epitope enhanced TARP peptides. scHLA-A2/peptide complexes were generated as described in materials and methods. The stability of the wild-type and modified complexes was monitored by reactivity with MAb W6/32 which recognizes properly folded complexes that contain peptide. Reactivity was monitored with Peroxidase-labeled anti-human Fab.
Stabilization of class I MHC carrying wild type and epitope enhanced TARP peptides on the cell surface. Flow cytometric analysis of RMAS-HHD cells which are TAP-mutant APCs stably transfected with HLA-A2. Cells were loaded with wild type TARP29-37, TARP29-37-9V, TARP29-37-3A and control Tax peptides. The stabilization of complexes on the cell surface was monitored by the reactivity of BB7.2, a MAb to HLA-A2. Detection was with FITC-labeled anti-mouse IgG.
Reactivity of D2 Fab with wild-type and epitope enhanced TARP peptides on the surface of cells. Flow cytometry analysis of RMAS-HHD cells that were loaded with TARP29-37, TARP29-37-9V, TARP29-37-3A and control Tax peptides and the binding of D2 Fab was monitored with PE-labeled anti-human Fab.



A. Flow cytometry binding analysis of D2 Fab-PE38 fusion to JY APCs. JY cells were loaded with TARP and control Tax and G9-209 peptides and the reactivity of the D2 Fab-PE38 fusion molecule was determined by using rabbit anti-PE38 antibodies. Detection was with FITC-anti rabbit IgG.
B-J. Flow cytometry binding analysis of D2 Fb-PE38 fusion to tumor cells. Binding of D2 Fab-PE38 to TARP positive and negative tumor cells was monitored by rabbit anti-PE38 antibodies. In B,E, and H binding to tumor cells was tested. In C,F, and I binding was to tumor cells pulsed with the TARP peptide. Monitoring of HLA-A2 expression was with BB7.2 (D,G,and J). Binding of PE38 fusion was detected with FITC-labeled anti rabbit IgG. Binding of BB7.2 was with FITC-labeled anti-mouse IgG.


Similar articles
-
Direct phenotypic analysis of human MHC class I antigen presentation: visualization, quantitation, and in situ detection of human viral epitopes using peptide-specific, MHC-restricted human recombinant antibodies.J Immunol. 2003 Apr 15;170(8):4349-61. doi: 10.4049/jimmunol.170.8.4349. J Immunol. 2003. PMID: 12682272
-
Effect of an immunotoxin to folate receptor beta on bleomycin-induced experimental pulmonary fibrosis.Clin Exp Immunol. 2010 Aug;161(2):348-56. doi: 10.1111/j.1365-2249.2010.04182.x. Epub 2010 Jun 9. Clin Exp Immunol. 2010. PMID: 20550546 Free PMC article.
-
A novel composite immunotoxin that suppresses rabies virus production by the infected cells.J Immunol Methods. 2010 Feb 28;353(1-2):78-86. doi: 10.1016/j.jim.2009.11.010. Epub 2009 Nov 22. J Immunol Methods. 2010. PMID: 19932697 Free PMC article.
-
Recombinant antibodies with MHC-restricted, peptide-specific, T-cell receptor-like specificity: new tools to study antigen presentation and TCR-peptide-MHC interactions.J Mol Recognit. 2003 Sep-Oct;16(5):324-32. doi: 10.1002/jmr.640. J Mol Recognit. 2003. PMID: 14523945 Review.
-
Cintredekin besudotox in treatment of malignant glioma.Expert Opin Biol Ther. 2008 Jun;8(6):805-12. doi: 10.1517/14712598.8.6.805. Expert Opin Biol Ther. 2008. PMID: 18476792 Review.
Cited by
-
TARP as antigen in cancer immunotherapy.Cancer Immunol Immunother. 2021 Nov;70(11):3061-3068. doi: 10.1007/s00262-021-02972-x. Epub 2021 May 29. Cancer Immunol Immunother. 2021. PMID: 34050774 Free PMC article. Review.
-
T cells engineered with a T cell receptor against the prostate antigen TARP specifically kill HLA-A2+ prostate and breast cancer cells.Proc Natl Acad Sci U S A. 2012 Sep 25;109(39):15877-81. doi: 10.1073/pnas.1209042109. Epub 2012 Sep 10. Proc Natl Acad Sci U S A. 2012. PMID: 23019373 Free PMC article.
-
Immunotherapy in prostate cancer: emerging strategies against a formidable foe.Vaccine. 2011 Sep 2;29(38):6485-97. doi: 10.1016/j.vaccine.2011.06.088. Epub 2011 Jul 7. Vaccine. 2011. PMID: 21741424 Free PMC article. Review.
-
Therapeutic Antibodies against Intracellular Tumor Antigens.Front Immunol. 2017 Aug 18;8:1001. doi: 10.3389/fimmu.2017.01001. eCollection 2017. Front Immunol. 2017. PMID: 28868054 Free PMC article. Review.
-
Targeting mutant p53-expressing tumours with a T cell receptor-like antibody specific for a wild-type antigen.Nat Commun. 2019 Nov 26;10(1):5382. doi: 10.1038/s41467-019-13305-z. Nat Commun. 2019. PMID: 31772160 Free PMC article.
References
-
- Saffran DC, Reiter RE, Jakobovits A, Witte ON. Target antigens for prostate cancer immunotherapy. Cancer Metastasis Rev. 1999;18:437–449. - PubMed
-
- Schroers R, Shen L, Rollins L, Xiao Z, Sonderstrup G, Slawin K, Huang XF, et al. Identification of MHC class II-restricted T-cell epitopes in prostate-specific membrane antigen. Clin.Cancer Res. 2003;9:3260–3271. - PubMed
-
- Dannull J, Diener PA, Prikler L, Furstenberger G, Cerny T, Schmid U, Ackermann DK, et al. Prostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate cancer. Cancer Res. 2000;60:5522–5528. - PubMed
-
- Kiessling A, Schmitz M, Stevanovic S, Weigle B, Holig K, Fussel M, Fussel S, et al. Prostate stem cell antigen: Identification of immunogenic peptides and assessment of reactive CD8+ T cells in prostate cancer patients. Int.J.Cancer. 2002;102:390–397. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

