Brachy-syndactyly caused by loss of Sfrp2 function
- PMID: 18446812
- PMCID: PMC2677682
- DOI: 10.1002/jcp.21483
Brachy-syndactyly caused by loss of Sfrp2 function
Abstract
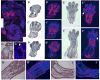
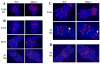
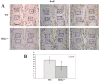
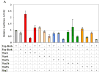
Wnt signaling pathways are regulated both at the intracellular and extracellular levels. During embryogenesis, the in vivo effects of the secreted frizzled-related protein (Sfrp) family of Wnt inhibitors are poorly understood. Here, we show that inactivation of Sfrp2 results in subtle limb defects in mice with mesomelic shortening and consistent shortening of all autopodal elements that is clinically manifested as brachydactyly. In addition, there is soft-tissue syndactyly of the hindlimb. The brachydactyly is caused by decreased chondrocyte proliferation and delayed differentiation in distal limb chondrogenic elements. These data suggest that Sfrp2 can regulate both chondrogenesis and regression of interdigital mesenchyme in distal limb. Sfrp2 can also repress canonical Wnt signaling by Wnt1, Wnt9a, and Wnt4 in vitro. Sfrp2-/- and TOPGAL/Sfrp2-/- mice have a mild increase in beta-catenin and beta-galactosidase staining, respectively, in some phalangeal elements. This however does not exclude a potential concurrent effect on non-canonical Wnt signaling in the growth plate. In combination with what is known about BMP and Wnt signaling in human brachydactylies, our data establish a critical role for Sfrp2 in proper distal limb formation and suggest SFPR2 could be a novel candidate gene for human brachy-syndactyly defects.
(c) 2008 Wiley-Liss, Inc.
Figures
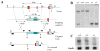

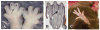

Similar articles
-
Dual roles of Wnt signaling during chondrogenesis in the chicken limb.Development. 2000 Jul;127(14):3141-59. doi: 10.1242/dev.127.14.3141. Development. 2000. PMID: 10862751
-
Syndactyly and preaxial synpolydactyly in the single Sfrp2 deleted mutant mice.Dev Dyn. 2008 Sep;237(9):2506-17. doi: 10.1002/dvdy.21655. Dev Dyn. 2008. PMID: 18729207
-
Sfrp2 is a transcriptional target of SREBP-1 in mouse chondrogenic cells.Mol Cell Biochem. 2015 Aug;406(1-2):163-71. doi: 10.1007/s11010-015-2434-y. Epub 2015 May 14. Mol Cell Biochem. 2015. PMID: 25971371
-
Early steps in limb patterning and chondrogenesis.Novartis Found Symp. 2001;232:23-36; discussion 36-46. doi: 10.1002/0470846658.ch3. Novartis Found Symp. 2001. PMID: 11277083 Review.
-
Secreted frizzled-related protein 2: a key player in noncanonical Wnt signaling and tumor angiogenesis.Cancer Metastasis Rev. 2021 Mar;40(1):191-203. doi: 10.1007/s10555-020-09941-3. Epub 2020 Nov 2. Cancer Metastasis Rev. 2021. PMID: 33140138 Free PMC article. Review.
Cited by
-
High-throughput screening of mouse gene knockouts identifies established and novel skeletal phenotypes.Bone Res. 2014 Oct 28;2:14034. doi: 10.1038/boneres.2014.34. eCollection 2014. Bone Res. 2014. PMID: 26273529 Free PMC article.
-
17 variants interaction of Wnt/β-catenin pathway associated with development of osteonecrosis of femoral head in Chinese Han population.Sci Rep. 2024 Mar 27;14(1):7301. doi: 10.1038/s41598-024-57929-8. Sci Rep. 2024. PMID: 38538713 Free PMC article.
-
Comparative effects of insulin pump and injection on gestational diabetes mellitus pregnancy outcomes and serum biomarkers.World J Clin Cases. 2024 Jun 26;12(18):3378-3384. doi: 10.12998/wjcc.v12.i18.3378. World J Clin Cases. 2024. PMID: 38983416 Free PMC article.
-
Differential effects of collagen prolyl 3-hydroxylation on skeletal tissues.PLoS Genet. 2014 Jan;10(1):e1004121. doi: 10.1371/journal.pgen.1004121. Epub 2014 Jan 23. PLoS Genet. 2014. PMID: 24465224 Free PMC article.
-
Phenotypic analysis of Myo10 knockout (Myo10tm2/tm2) mice lacking full-length (motorized) but not brain-specific headless myosin X.Sci Rep. 2019 Jan 24;9(1):597. doi: 10.1038/s41598-018-37160-y. Sci Rep. 2019. PMID: 30679680 Free PMC article.
References
-
- Baur ST, Mai JJ, Dymecki SM. Combinatorial signaling through BMP receptor IB and GDF5: shaping of the distal mouse limb and the genetics of distal limb diversity. Development. 2000;127(3):605–619. - PubMed
-
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172(1):126–138. - PubMed
-
- Bodine PV, Billiard J, Moran RA, Ponce-de-Leon H, McLarney S, Mangine A, Scrimo MJ, Bhat RA, Stauffer B, Green J, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005;96(6):1212–1230. - PubMed
-
- Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18(5):1222–1237. - PubMed
-
- Chimal-Monroy J, Montero JA, Ganan Y, Macias D, Garcia-Porrero JA, Hurle JM. Comparative analysis of the expression and regulation of Wnt5a, Fz4, and Frzb1 during digit formation and in micromass cultures. Dev Dyn. 2002;224(3):314–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

