Magnetic microposts for mechanical stimulation of biological cells: fabrication, characterization, and analysis
- PMID: 18447536
- PMCID: PMC2809695
- DOI: 10.1063/1.2906228
Magnetic microposts for mechanical stimulation of biological cells: fabrication, characterization, and analysis
Abstract
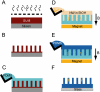
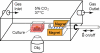
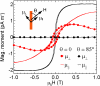
Cells use force as a mechanical signal to sense and respond to their microenvironment. Understanding how mechanical forces affect living cells requires the development of tool sets that can apply nanoscale forces and also measure cellular traction forces. However, there has been a lack of techniques that integrate actuation and sensing components to study force as a mechanical signal. Here, we describe a system that uses an array of elastomeric microposts to apply external forces to cells through cobalt nanowires embedded inside the microposts. We first biochemically treat the posts' surfaces to restrict cell adhesion to the posts' tips. Then by applying a uniform magnetic field (B<0.3 T), we induce magnetic torque on the nanowires that is transmitted to a cell's adhesion site as an external force. We have achieved external forces of up to 45 nN, which is in the upper range of current nanoscale force-probing techniques. Nonmagnetic microposts, similarly prepared but without nanowires, surround the magnetic microposts and are used to measure the traction forces and changes in cell mechanics. We record the magnitude and direction of the external force and the traction forces by optically measuring the deflection of the microposts, which linearly deflect as cantilever springs. With this approach, we can measure traction forces before and after force stimulation in order to monitor cellular response to forces. We present the fabrication methods, magnetic force characterization, and image analysis techniques used to achieve the measurements.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

