High-throughput SHAPE analysis reveals structures in HIV-1 genomic RNA strongly conserved across distinct biological states
- PMID: 18447581
- PMCID: PMC2689691
- DOI: 10.1371/journal.pbio.0060096
High-throughput SHAPE analysis reveals structures in HIV-1 genomic RNA strongly conserved across distinct biological states
Abstract
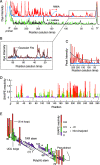
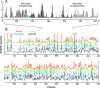
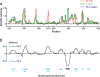
Replication and pathogenesis of the human immunodeficiency virus (HIV) is tightly linked to the structure of its RNA genome, but genome structure in infectious virions is poorly understood. We invent high-throughput SHAPE (selective 2'-hydroxyl acylation analyzed by primer extension) technology, which uses many of the same tools as DNA sequencing, to quantify RNA backbone flexibility at single-nucleotide resolution and from which robust structural information can be immediately derived. We analyze the structure of HIV-1 genomic RNA in four biologically instructive states, including the authentic viral genome inside native particles. Remarkably, given the large number of plausible local structures, the first 10% of the HIV-1 genome exists in a single, predominant conformation in all four states. We also discover that noncoding regions functioning in a regulatory role have significantly lower (p-value < 0.0001) SHAPE reactivities, and hence more structure, than do viral coding regions that function as the template for protein synthesis. By directly monitoring protein binding inside virions, we identify the RNA recognition motif for the viral nucleocapsid protein. Seven structurally homologous binding sites occur in a well-defined domain in the genome, consistent with a role in directing specific packaging of genomic RNA into nascent virions. In addition, we identify two distinct motifs that are targets for the duplex destabilizing activity of this same protein. The nucleocapsid protein destabilizes local HIV-1 RNA structure in ways likely to facilitate initial movement both of the retroviral reverse transcriptase from its tRNA primer and of the ribosome in coding regions. Each of the three nucleocapsid interaction motifs falls in a specific genome domain, indicating that local protein interactions can be organized by the long-range architecture of an RNA. High-throughput SHAPE reveals a comprehensive view of HIV-1 RNA genome structure, and further application of this technology will make possible newly informative analysis of any RNA in a cellular transcriptome.
Conflict of interest statement
Figures


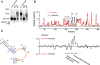





References
-
- Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor (New York): Cold Spring Harbor Press; 1997. 843 - PubMed
-
- Frankel AD, Young JAT. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. - PubMed
-
- Berkowitz R, Fisher J, Goff SP. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. - PubMed
-
- Rein A. Take two. Nature Struct Mol Biol. 2004;11:1034–1035. - PubMed
-
- D'Souza V, Summers MF. How retroviruses select their genomes. Nat Rev Microbiol. 2005;3:643–655. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

