Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites
- PMID: 18447583
- PMCID: PMC2689695
- DOI: 10.1371/journal.pbio.0060103
Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites
Abstract
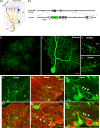
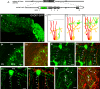
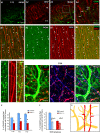
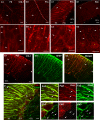
The geometric and subcellular organization of axon arbors distributes and regulates electrical signaling in neurons and networks, but the underlying mechanisms have remained elusive. In rodent cerebellar cortex, stellate interneurons elaborate characteristic axon arbors that selectively innervate Purkinje cell dendrites and likely regulate dendritic integration. We used GFP BAC transgenic reporter mice to examine the cellular processes and molecular mechanisms underlying the development of stellate cell axons and their innervation pattern. We show that stellate axons are organized and guided towards Purkinje cell dendrites by an intermediate scaffold of Bergmann glial (BG) fibers. The L1 family immunoglobulin protein Close Homologue of L1 (CHL1) is localized to apical BG fibers and stellate cells during the development of stellate axon arbors. In the absence of CHL1, stellate axons deviate from BG fibers and show aberrant branching and orientation. Furthermore, synapse formation between aberrant stellate axons and Purkinje dendrites is reduced and cannot be maintained, leading to progressive atrophy of axon terminals. These results establish BG fibers as a guiding scaffold and CHL1 a molecular signal in the organization of stellate axon arbors and in directing their dendritic innervation.
Conflict of interest statement
Figures
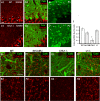
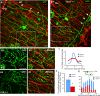
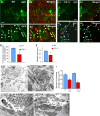
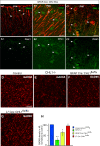
Comment in
-
Cellular conductors: glial cells as guideposts during neural circuit development.PLoS Biol. 2008 Apr 29;6(4):e112. doi: 10.1371/journal.pbio.0060112. PLoS Biol. 2008. PMID: 18447586 Free PMC article.
References
-
- Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. - PubMed
-
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. - PubMed
-
- Schaefer AT, Larkum ME, Sakmann B, Roth A. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol. 2003;89:3143–3154. - PubMed
-
- London M, Hausser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

