Antiretroviral therapy and central nervous system HIV type 1 infection
- PMID: 18447615
- PMCID: PMC2628635
- DOI: 10.1086/533419
Antiretroviral therapy and central nervous system HIV type 1 infection
Abstract
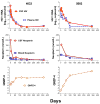
Central nervous system (CNS) human immunodeficiency virus type 1 (HIV-1) infection begins during primary viremia and continues throughout the course of untreated systemic infection. Although frequently accompanied by local inflammatory reactions detectable in cerebrospinal fluid (CSF), CNS HIV-1 infection usually is not clinically apparent. In a minority of patients, CNS HIV-1 infection evolves into encephalitis during the late stages of systemic infection, which compromises brain function and presents clinically as acquired immunodeficiency syndrome dementia complex (ADC). Combination antiretroviral therapy (ART) has had a major impact on all aspects of CNS HIV-1 infection and disease. In those with asymptomatic infection, ART usually effectively suppresses HIV-1 in CSF and markedly reduces the incidence of symptomatic ADC. In those presenting with ADC, ART characteristically prevents neurological progression and leads to variable, and at times substantial, recovery. Similarly, treatment has reduced CNS opportunistic infections. With better control of these severe disorders, attention has turned to the possible consequences of chronic silent infection and the issue of whether indolent, low-grade brain injury might require earlier treatment intervention.
Figures

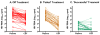
References
-
- Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–9. - PubMed
-
- Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–45. - PubMed
-
- Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

