Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides
- PMID: 18447912
- PMCID: PMC2408928
- DOI: 10.1186/1471-2407-8-122
Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides
Abstract
Background: Among the most prominent metabolic alterations in cancer cells are the increase in glucose consumption and the conversion of glucose to lactic acid via the reduction of pyruvate even in the presence of oxygen. This phenomenon, known as aerobic glycolysis or the Warburg effect, may provide a rationale for therapeutic strategies that inhibit tumour growth by administration of a ketogenic diet with average protein but low in carbohydrates and high in fat enriched with omega-3 fatty acids and medium-chain triglycerides (MCT).
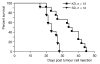
Methods: Twenty-four female NMRI nude mice were injected subcutaneously with tumour cells of the gastric adenocarcinoma cell line 23132/87. The animals were then randomly split into two feeding groups and fed either a ketogenic diet (KD group; n = 12) or a standard diet (SD group; n = 12) ad libitum. Experiments were ended upon attainment of the target tumor volume of 600 mm3 to 700 mm3. The two diets were compared based on tumour growth and survival time (interval between tumour cell injection and attainment of target tumour volume).
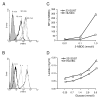
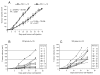
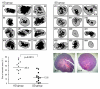
Results: The ketogenic diet was well accepted by the KD mice. The tumour growth in the KD group was significantly delayed compared to that in the SD group. Tumours in the KD group reached the target tumour volume at 34.2 +/- 8.5 days versus only 23.3 +/- 3.9 days in the SD group. After day 20, tumours in the KD group grew faster although the differences in mean tumour growth continued significantly. Importantly, they revealed significantly larger necrotic areas than tumours of the SD group and the areas with vital tumour cells appear to have had fewer vessels than tumours of the SD group. Viable tumour cells in the border zone surrounding the necrotic areas of tumours of both groups exhibited a glycolytic phenotype with expression of glucose transporter-1 and transketolase-like 1 enzyme.
Conclusion: Application of an unrestricted ketogenic diet enriched with omega-3 fatty acids and MCT delayed tumour growth in a mouse xenograft model. Further studies are needed to address the impact of this diet on other tumour-relevant functions such as invasive growth and metastasis.
Figures

References
-
- Wittig R, Coy JF. The Role of Glucose Metabolism and Glucose-Associated Signalling in Cancer. Perspectives in Medicinal Chemistry. 2007;1:64–82. http://la-press.com/cr_data/files/f_PMC-1-Coy-et-al_490.pdf - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

