Hypoxia-inducible factor-1alpha is a critical mediator of hypoxia induced apoptosis in cardiac H9c2 and kidney epithelial HK-2 cells
- PMID: 18447926
- PMCID: PMC2387135
- DOI: 10.1186/1471-2261-8-9
Hypoxia-inducible factor-1alpha is a critical mediator of hypoxia induced apoptosis in cardiac H9c2 and kidney epithelial HK-2 cells
Abstract
Background: Hypoxia inducible factor-1 (HIF-1) is a transcription factor that functions to maintain cellular homeostasis in response to hypoxia. There is evidence that HIF-1 can also trigger apoptosis, possibly when cellular responses are inadequate to meet energy demands under hypoxic conditions.
Methods: Cardiac derived H9c2 and renal tubular epithelial HK-2 cells expressing either the wild type oxygen regulated subunit of HIF-1 (pcDNA3-Hif-1alpha) or a dominant negative version that lacked both DNA binding and transactivation domains (pcDNA3-DN-Hif-1alpha), were maintained in culture and exposed to hypoxia. An RNA interference approach was also employed to selectively knockdown expression of Hif-1alpha. Apoptosis was analyzed in both H9c2 and HK-2 cells by Hoechst and TUNEL staining, caspase 3 activity assays and activation of pro-apoptotic Bcl2 family member Bax.
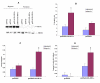
Results: Overexpression of pcDNA3-DN-Hif-1alpha led to a significant reduction in hypoxia -induced apoptosis (17 +/- 2%, P < 0.01) in H9c2 cells compared to both control-transfected and wild type Hif-1alpha transfected cells. Moreover, selective ablation of HIF-1alpha protein expression by RNA interference in H9c2 cells led to 55% reduction of caspase 3 activity and 46% reduction in the number of apoptotic cells as determined by Hoechst 33258 staining, after hypoxia. Finally, upregulation of the pro-apoptotic protein, Bax, was found in H9c2 cells overexpressing full-length pcDNA3-HA-HIF-1alpha exposed to hypoxia. In HK-2 cells overexpression of wild-type Hif-1alpha led to a two-fold increase in Hif-1alpha levels during hypoxia. This resulted in a 3.4-fold increase in apoptotic cells and a concomitant increase in caspase 3 activity during hypoxia when compared to vector transfected control cells. HIF-1alpha also induced upregulation of Bax in HK-2 cells. In addition, introduction of dominant negative Hif-1alpha constructs in both H9c2 and HK-2 -cells led to decreased active Bax expression.
Conclusion: These data demonstrate that HIF-1alpha is an important component of the apoptotic signaling machinery in the two cell types.
Figures
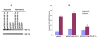


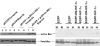
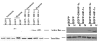
Similar articles
-
miR-20b-5p attenuates hypoxia-induced apoptosis in cardiomyocytes via the HIF-1α/NF-κB pathway.Acta Biochim Biophys Sin (Shanghai). 2020 Sep 8;52(9):927-934. doi: 10.1093/abbs/gmaa056. Acta Biochim Biophys Sin (Shanghai). 2020. PMID: 32510153
-
Xinnaotongluo liquid protects H9c2 cells against hypoxic damage through IRF2/HIF-1α-mediated oxidation, inflammation, and apoptosis.Histol Histopathol. 2025 Aug;40(8):1287-1294. doi: 10.14670/HH-18-855. Epub 2024 Nov 28. Histol Histopathol. 2025. PMID: 39691995
-
[Effects of hypoxia-inducible factor 1α on hypoxic tolerance of human amniotic mesenchymal stem cells].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Mar 15;32(3):264-269. doi: 10.7507/1002-1892.201710104. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 29806273 Free PMC article. Chinese.
-
Contribution of hypoxia to Alzheimer's disease: is HIF-1alpha a mediator of neurodegeneration?Cell Mol Life Sci. 2009 Nov;66(22):3555-63. doi: 10.1007/s00018-009-0141-0. Epub 2009 Sep 11. Cell Mol Life Sci. 2009. PMID: 19763399 Free PMC article. Review.
-
Unlocking mammalian regeneration through hypoxia inducible factor one alpha signaling.Biomaterials. 2021 Feb;269:120646. doi: 10.1016/j.biomaterials.2020.120646. Epub 2021 Jan 9. Biomaterials. 2021. PMID: 33493769 Free PMC article. Review.
Cited by
-
The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor α-mediated signaling in cancer cells and in cardiomyocytes.PLoS One. 2011 Jan 31;6(1):e16631. doi: 10.1371/journal.pone.0016631. PLoS One. 2011. PMID: 21304949 Free PMC article.
-
Engineered myocardium model to study the roles of HIF-1α and HIF1A-AS1 in paracrine-only signaling under pathological level oxidative stress.Acta Biomater. 2017 Aug;58:323-336. doi: 10.1016/j.actbio.2017.06.023. Epub 2017 Jun 16. Acta Biomater. 2017. PMID: 28629892 Free PMC article.
-
Intracellular prostaglandin E2 contributes to hypoxia-induced proximal tubular cell death.Sci Rep. 2021 Mar 29;11(1):7047. doi: 10.1038/s41598-021-86219-w. Sci Rep. 2021. PMID: 33782420 Free PMC article.
-
Antiangiogenic Effect of Alkaloids.Oxid Med Cell Longev. 2019 Apr 21;2019:9475908. doi: 10.1155/2019/9475908. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31178979 Free PMC article. Review.
-
Bicalutamide Elicits Renal Damage by Causing Mitochondrial Dysfunction via ROS Damage and Upregulation of HIF-1.Int J Mol Sci. 2020 May 11;21(9):3400. doi: 10.3390/ijms21093400. Int J Mol Sci. 2020. PMID: 32403414 Free PMC article.
References
-
- Brunelle JK, Santore MT, Budinger GR, Tang Y, Barret TA, Zong WX, Kandel E, Keith B, Simon C, Thompson CB, Hay N, Chandel NS. C-Myc sensitization to oxygen deprivation-induced cell death is dependent on Bax/Bak, but is independent of p53 and hypoxia inducible factor-1. J Biol Chem. 2004;279:4305–4312. doi: 10.1074/jbc.M312241200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

