Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions
- PMID: 18448100
- PMCID: PMC3018150
- DOI: 10.1016/j.expneurol.2008.01.031
Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions
Abstract
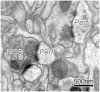
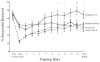
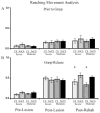
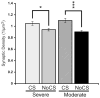
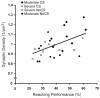
Cortical stimulation (CS) as a means to modulate regional activity and excitability in cortex is emerging as a promising approach for facilitating rehabilitative interventions after brain damage, including stroke. In this study, we investigated whether CS-induced functional improvements are linked with synaptic plasticity in peri-infarct cortex and vary with the severity of impairments. Adult rats that were proficient in skilled reaching received subtotal unilateral ischemic sensorimotor cortex (SMC) lesions and implantation of chronic epidural electrodes over remaining motor cortex. Based on the initial magnitude of reaching deficits, rats were divided into severely and moderately impaired subgroups. Beginning two weeks post-surgery, rats received 100 Hz cathodal CS at 50% of movement thresholds or no-stimulation control procedures (NoCS) during 18 days of rehabilitative training on a reaching task. Stereological electron microscopy methods were used to quantify axodendritic synapse subtypes in motor cortical layer V underlying the electrode. In moderately, but not severely impaired rats, CS significantly enhanced recovery of reaching success. Sensitive movement analyses revealed that CS partially normalized reaching movements in both impairment subgroups compared to NoCS. Additionally, both CS subgroups had significantly greater density of axodendritic synapses and moderately impaired CS rats had increases in presumed efficacious synapse subtypes (perforated and multiple synapses) in stimulated cortex compared to NoCS. Synaptic density was positively correlated with post-rehabilitation reaching success. In addition to providing further support that CS can promote functional recovery, these findings suggest that CS-induced functional improvements may be mediated by synaptic structural plasticity in stimulated cortex.
Figures



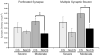
References
-
- Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–1782. - PubMed
-
- Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol. 2006;200:356–370. - PubMed
-
- Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. - PubMed
-
- Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–788. - PubMed
-
- Adkins-Muir DL, Luke L, Fowler B, Jones TA. Electrical stimulation of the peri-lesion cortical surface during motor skills training improves behavioral function after ischemic lesions of the sensorimotor cortex in adult rats. Soc Neurosci Abstr. 2002;28:489.4.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

