Benzo(a)pyrene-caused increased G1-S transition requires the activation of c-Jun through p53-dependent PI-3K/Akt/ERK pathway in human embryo lung fibroblasts
- PMID: 18448277
- PMCID: PMC3759236
- DOI: 10.1016/j.toxlet.2008.03.012
Benzo(a)pyrene-caused increased G1-S transition requires the activation of c-Jun through p53-dependent PI-3K/Akt/ERK pathway in human embryo lung fibroblasts
Abstract
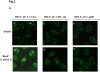
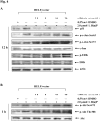
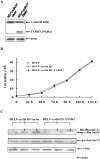
Benzo(a)pyrene (B(a)P) is a potent lung carcinogen mainly derived from tobacco smoking and environmental contamination, however, the molecular mechanisms by which it accelerates the cell cycle progression and induces the abnormal cell proliferation are still far away from understood. Our current analysis of human embryo lung fibroblasts (HELF) showed that B(a)P exposure was able to promote cell cycle G(1)-S phase transition. This effect was correlated with c-Jun activation because inhibition of c-Jun by its dominant negative mutant (TAM67) reversed B(a)P action on cell cycle with the down-regulation of expression of cyclin D1, pRb and E2F1. Further study found that overexpression of dominant negative mutants of, PI-3K or Akt, dramatically reduced B(a)P-induced the activation of c-Jun and extracellular signaling regulated kinase (ERK), but not c-Jun NH2 terminal kinase (JNK). Inhibition of p53 by either its inhibitor pifithrin-alpha or p53 siRNA markedly increased B(a)P-induced the activation of c-Jun, Akt and ERK in this context. Take together, our results indicate that c-Jun activation by p53-dependent PI-3K/Akt/ERK pathway is responsible for B(a)P-induced cell cycle alternations in human embryo lung fibroblasts.
Figures








Similar articles
-
Phosphatidylinositol-3 kinase/Akt/p70S6K/AP-1 signaling pathway mediated benzo(a)pyrene-induced cell cycle alternation via cell cycle regulatory proteins in human embryo lung fibroblasts.Toxicol Lett. 2007 Apr 5;170(1):30-41. doi: 10.1016/j.toxlet.2007.02.008. Epub 2007 Feb 21. Toxicol Lett. 2007. PMID: 17383120
-
[c-Jun NH2-terminal kinase and extracellular signal-regulated protein kinase signaling pathways in regulation of benzo(a)pyrene-induced c-Jun activation in human embryo lung fibroblasts].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007 Jul;25(7):385-8. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007. PMID: 17908424 Chinese.
-
Vitamin C inhibits benzo[a]pyrene-induced cell cycle changes partly via cyclin D1/E2F pathway in human embryo lung fibroblasts.Biomed Environ Sci. 2006 Jun;19(3):239-44. Biomed Environ Sci. 2006. PMID: 16944783
-
[ERK and JNK/AP-1 pathways involved in benzo(a)pyrene induced cell cycle changes in human embryo lung fibroblasts].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2006 Feb;24(2):72-6. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2006. PMID: 16600107 Chinese.
-
Regulation of cell cycle entry and G1 progression by CSF-1.Mol Reprod Dev. 1997 Jan;46(1):11-8. doi: 10.1002/(SICI)1098-2795(199701)46:1<11::AID-MRD3>3.0.CO;2-U. Mol Reprod Dev. 1997. PMID: 8981358 Review.
Cited by
-
Activation of MAPK and Cyclin D1/CDK4 in Malignant Transformation of Human Embryonic Lung Fibroblasts Induced by Silica and Benzopyrene.Asian Pac J Cancer Prev. 2020 Feb 1;21(2):295-300. doi: 10.31557/APJCP.2020.21.2.295. Asian Pac J Cancer Prev. 2020. PMID: 32102502 Free PMC article.
-
Molecular alterations associated with chronic exposure to cigarette smoke and chewing tobacco in normal oral keratinocytes.Cancer Biol Ther. 2018;19(9):773-785. doi: 10.1080/15384047.2018.1470724. Epub 2018 May 29. Cancer Biol Ther. 2018. PMID: 29723088 Free PMC article.
-
Influence of cell cycle on responses of MCF-7 cells to benzo[a]pyrene.BMC Genomics. 2011 Jun 29;12:333. doi: 10.1186/1471-2164-12-333. BMC Genomics. 2011. PMID: 21714911 Free PMC article.
-
The Anticancer Effects of the Garlic Organosulfide Diallyl Trisulfide through the Attenuation of B[a]P-Induced Oxidative Stress, AhR Expression, and DNA Damage in Human Premalignant Breast Epithelial (MCF-10AT1) Cells.Int J Mol Sci. 2024 Jan 11;25(2):923. doi: 10.3390/ijms25020923. Int J Mol Sci. 2024. PMID: 38255999 Free PMC article.
-
Hepatitis B spliced protein (HBSP) promotes the carcinogenic effects of benzo [alpha] pyrene by interacting with microsomal epoxide hydrolase and enhancing its hydrolysis activity.BMC Cancer. 2014 Apr 23;14:282. doi: 10.1186/1471-2407-14-282. BMC Cancer. 2014. PMID: 24758376 Free PMC article.
References
-
- Andrysík Z, Vondrácek J, Machala M, Krcmár P, Svihálková-Sindlerová L, Kranz A, Weiss C, Faust D, Kozubík A, Dietrich C. The aryl hydrocarbon receptor-dependent deregulation of cell cycle control induced by polycyclic aromatic hydrocarbons in rat liver epithelial cells. Mutat Res. 2007;615:87–97. - PubMed
-
- Chramostova K, Vondracek J, Sindlerova L, Vojtesek B, Kozubik A, Machala M. Polycyclic aromatic hydrocarbons modulate cell proliferation in rat hepatic epithelial stem-like WB-F344 cells. Toxicol Appl Pharmacol. 2004;196:136–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

