Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve
- PMID: 18448399
- PMCID: PMC10615646
- DOI: 10.1016/j.actbio.2008.03.014
Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve
Abstract
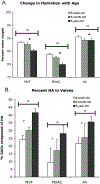
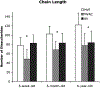
This study characterized valve proteoglycan and glycosaminoglycan composition during development and aging. This knowledge is important for the development of age-specific tissue-engineered heart valves as well as treatments for age-specific valvulopathies. Aortic valves and mitral valves from first-third trimester, 6-week, 6-month and 6-year-old pigs were examined using immunohistochemistry for versican, biglycan, decorin and hyaluronan, as well as elastin and fibrillin. The fine structure of glycosaminoglycans was examined by fluorophore-assisted carbohydrate electrophoresis. Decorin expression was strongest in the 6-year-old valves, particularly in the aortic valve spongiosa. The quantity of iduronate was also highest in the 6-year-old valves. The central tensile-loading region of the anterior mitral leaflet demonstrated reduced glycosaminoglycan content, chain length and hydration and a larger fraction of 4-sulfated iduronate and lower fraction of 6-sulfation. With age, the anterior leaflet center showed a further increase in 4-sulfated iduronate and decrease in 6-sulfation. In contrast, the anterior leaflet free edge showed decreased iduronate and 4-sulfated glucuronate content with age. The young aortic valve was similar to the mitral valve free edge with a higher concentration of glycosaminoglycans and 6-rather than 4-sulfation, but aged to resemble the mitral anterior leaflet center, with an increase in 4-sulfated iduronate content and a decrease in the 6-sulfation fraction. Elastin and fibrillin often co-localized with the proteoglycans studied, but elastin co-localized most specifically with versican. In conclusion, composition and fine structure changes in valve proteoglycans and glycosaminoglycans with age are complex and distinct within valve type, histological layers and regions of different mechanical loading.
Figures





References
-
- Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit. Rev. Eukaryot. Gene Expr 2004;14(3):203–34. - PubMed
-
- Rothenburger M, Volker W, Vischer P, Glasmacher B, Scheld HH, Deiwick M. Ultrastructure of proteoglycans in tissue-engineered cardiovascular structures. Tissue Eng. 2002. Dec;8(6):1049–56. - PubMed
-
- Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006. Mar 14;113(10):1344–52. - PubMed
-
- Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004. Jul;14(7):621–33. - PubMed
-
- Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J. Am. Coll. Cardiol. 2003. Jul 16;42(2):271–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

