Hepatitis B virus HBx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential
- PMID: 18448529
- PMCID: PMC2446973
- DOI: 10.1128/JVI.00154-08
Hepatitis B virus HBx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential
Abstract
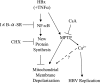
Over 350 million people are chronically infected with hepatitis B virus (HBV), and a significant number of chronically infected individuals develop primary liver cancer. HBV encodes seven viral proteins, including the nonstructural X (HBx) protein. The results of studies with immortalized or transformed cells and with HBx-transgenic mice demonstrated that HBx can interact with mitochondria. However, no studies with normal hepatocytes have characterized the precise mitochondrial localization of HBx or the effect of HBx on mitochondrial physiology. We have used cultured primary rat hepatocytes as a model system to characterize the mitochondrial localization of HBx and the effect of HBx expression on mitochondrial physiology. We now show that a fraction of HBx colocalizes with density-gradient-purified mitochondria and associates with the outer mitochondrial membrane. We also demonstrate that HBx regulates mitochondrial membrane potential in hepatocytes and that this function of HBx varies depending on the status of NF-kappaB activity. In primary rat hepatocytes, HBx activation of NF-kappaB prevented mitochondrial membrane depolarization; however, when NF-kappaB activity was inhibited, HBx induced membrane depolarization through modulation of the mitochondrial permeability transition pore. Collectively, these results define potential pathways through which HBx may act in order to modulate mitochondrial physiology, thereby altering many cellular activities and ultimately contributing to the development of HBV-associated liver cancer.
Figures
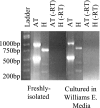



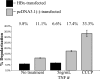
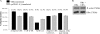

References
-
- Andrisani, O., and S. Barnabas. 1999. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis. Int. J. Oncol. 151-8. - PubMed
-
- Anflous, K., O. Blondel, A. Bernart, M. Khrestchatisky, and R. Ventura-Clapier. 1998. Characterization of rat porin isoforms: cloning of a cardiac type-3 variant encoding an additional methionine at its putative N-terminal region. Biochim. Biophys. Acta 139947-50. - PubMed
-
- Beasley, R., C.-C. Lin, L.-Y. Hwang, and C.-S. Chien. 1981. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22 707 men in Taiwan. Lancet ii1129-1133. - PubMed
-
- Bernardi, P. 1999. Mitochondrial transport and cations: channels, exchangers, and permeability transition. Physiol. Rev. 791127-1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

