The UL25 gene product of herpes simplex virus type 1 is involved in uncoating of the viral genome
- PMID: 18448531
- PMCID: PMC2447045
- DOI: 10.1128/JVI.00257-08
The UL25 gene product of herpes simplex virus type 1 is involved in uncoating of the viral genome
Abstract
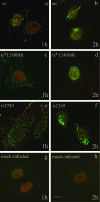
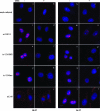
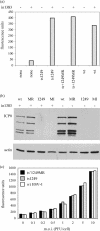
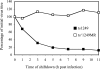
Studies on the herpes simplex virus type 1 UL25-null mutant KUL25NS have shown that the capsid-associated UL25 protein is required at a late stage in the encapsidation of viral DNA. Our previous work on UL25 with the UL25 temperature-sensitive (ts) mutant ts1204 also implicated UL25 in a role at very early times in the virus growth cycle, possibly at the stage of penetration of the host cell. We have reexamined this mutant and discovered that it had an additional ts mutation elsewhere in the genome. The ts1204 UL25 mutation was transferred into wild-type (wt) virus DNA, and the UL25 mutant ts1249 was isolated and characterized to clarify the function of UL25 at the initial stages of virus infection. Indirect immunofluorescence assays and in situ hybridization analysis of virus-infected cells revealed that the mutant ts1249 was not impaired in penetration of the host cell but had an uncoating defect at the nonpermissive temperature. When ts1249-infected cells were incubated initially at the permissive temperature to allow uncoating of the viral genome and subsequently transferred to the restrictive temperature, a DNA-packaging defect was evident. The results suggested that ts1249, like KUL25NS, had a block at a late stage of DNA packaging and that the packaged genome was shorter than the full-length genome. Examination of ts1249 capsids produced at the nonpermissive temperature revealed that, in comparison with wt capsids, they contained reduced amounts of UL25 protein, thereby providing a possible explanation for the failure of ts1249 to package full-length viral DNA.
Figures






Similar articles
-
The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA.J Virol. 1998 Feb;72(2):1060-70. doi: 10.1128/JVI.72.2.1060-1070.1998. J Virol. 1998. PMID: 9445000 Free PMC article.
-
Herpes simplex virus type 1 DNA-packaging protein UL17 is required for efficient binding of UL25 to capsids.J Virol. 2006 Mar;80(5):2118-26. doi: 10.1128/JVI.80.5.2118-2126.2006. J Virol. 2006. PMID: 16474120 Free PMC article.
-
Packaging of genomic and amplicon DNA by the herpes simplex virus type 1 UL25-null mutant KUL25NS.J Virol. 2001 Nov;75(22):10755-65. doi: 10.1128/JVI.75.22.10755-10765.2001. J Virol. 2001. PMID: 11602717 Free PMC article.
-
Involvement of Terminase Complex in Herpes Simplex Virus Mature Virion Egress.Curr Protein Pept Sci. 2022;23(2):105-113. doi: 10.2174/1389203723666220217144432. Curr Protein Pept Sci. 2022. PMID: 35176987 Review.
-
Molecular frustration: a hypothesis for regulation of viral infections.Trends Microbiol. 2024 Jan;32(1):17-26. doi: 10.1016/j.tim.2023.07.003. Epub 2023 Jul 27. Trends Microbiol. 2024. PMID: 37507296 Review.
Cited by
-
Role of the UL25 protein in herpes simplex virus DNA encapsidation.J Virol. 2009 Jan;83(1):47-57. doi: 10.1128/JVI.01889-08. Epub 2008 Oct 22. J Virol. 2009. PMID: 18945788 Free PMC article.
-
A Nuclear localization signal in herpesvirus protein VP1-2 is essential for infection via capsid routing to the nuclear pore.J Virol. 2012 Sep;86(17):8998-9014. doi: 10.1128/JVI.01209-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718835 Free PMC article.
-
Targeting of viral capsids to nuclear pores in a cell-free reconstitution system.Traffic. 2014 Nov;15(11):1266-81. doi: 10.1111/tra.12209. Epub 2014 Sep 12. Traffic. 2014. PMID: 25131140 Free PMC article.
-
Herpesvirus transport to the nervous system and back again.Annu Rev Microbiol. 2012;66:153-76. doi: 10.1146/annurev-micro-092611-150051. Epub 2012 Jun 15. Annu Rev Microbiol. 2012. PMID: 22726218 Free PMC article. Review.
-
The C terminus of the large tegument protein pUL36 contains multiple capsid binding sites that function differently during assembly and cell entry of herpes simplex virus.J Virol. 2012 Apr;86(7):3682-700. doi: 10.1128/JVI.06432-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258258 Free PMC article.
References
-
- Addison, C., F. J. Rixon, J. W. Palfreyman, M. O'Hara, and V. G. Preston. 1984. Characterization of a herpes simplex virus type-1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology 138246-259. - PubMed
-
- Baines, J. D., and S. K. Weller. 2005. Cleavage and packaging of herpes simplex virus 1 DNA, p. 135-150. In C. Catalano (ed.), Viral genome packaging machines. Kluwer Academic/Plenum Publishers, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

