Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing
- PMID: 18448536
- PMCID: PMC2447085
- DOI: 10.1128/JVI.00502-08
Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing
Abstract
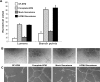
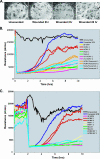
Human cytomegalovirus (HCMV) is implicated in the acceleration of a number of vascular diseases including transplant vascular sclerosis (TVS), the lesion associated with chronic rejection (CR) of solid organ transplants. Although the virus persists in the allograft throughout the course of disease, few cells are directly infected by CMV. This observation is in contrast to the global effects that CMV has on the acceleration of TVS/CR, suggesting that CMV infection indirectly promotes the vascular disease process. Recent transcriptome analysis of CMV-infected heart allografts indicates that the virus induces cytokines and growth factors associated with angiogenesis (AG) and wound healing (WH), suggesting that CMV may accelerate TVS/CR through the induction and secretion of AG/WH factors from infected cells. We analyzed virus-free supernatants from HCMV-infected cells (HCMV secretomes) for growth factors, by mass spectrometry and immunoassays, and found that the HCMV secretome contains over 1,000 cellular proteins, many of which are involved in AG/WH. Importantly, functional assays demonstrated that CMV but not herpes simplex virus secretomes not only induce AG/WH but also promote neovessel stabilization and endothelial cell survival for 2 weeks. These findings suggest that CMV acceleration of TVS occurs through virus-induced growth factors and cytokines in the CMV secretome.
Figures

Similar articles
-
Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing.Curr Top Microbiol Immunol. 2008;325:397-415. doi: 10.1007/978-3-540-77349-8_22. Curr Top Microbiol Immunol. 2008. PMID: 18637518 Free PMC article. Review.
-
IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin.Blood. 2011 Jan 6;117(1):352-61. doi: 10.1182/blood-2010-06-291245. Epub 2010 Oct 7. Blood. 2011. PMID: 20930069 Free PMC article.
-
The role of angiogenic and wound repair factors during CMV-accelerated transplant vascular sclerosis in rat cardiac transplants.Am J Transplant. 2008 Feb;8(2):277-87. doi: 10.1111/j.1600-6143.2007.02062.x. Epub 2007 Dec 18. Am J Transplant. 2008. PMID: 18093265
-
Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants.Transplantation. 2002 Mar 15;73(5):679-88. doi: 10.1097/00007890-200203150-00005. Transplantation. 2002. PMID: 11907411
-
Cytomegalovirus infection and cardiac allograft vasculopathy.Transpl Infect Dis. 1999 Jun;1(2):115-26. doi: 10.1034/j.1399-3062.1999.010205.x. Transpl Infect Dis. 1999. PMID: 11428979 Review.
Cited by
-
Human cytomegalovirus linked to stroke in a Chinese population.CNS Neurosci Ther. 2012 Jun;18(6):457-60. doi: 10.1111/j.1755-5949.2012.00326.x. CNS Neurosci Ther. 2012. PMID: 22672297 Free PMC article.
-
US28: HCMV's Swiss Army Knife.Viruses. 2018 Aug 20;10(8):445. doi: 10.3390/v10080445. Viruses. 2018. PMID: 30127279 Free PMC article. Review.
-
The life cycle and pathogenesis of human cytomegalovirus infection: lessons from proteomics.Expert Rev Proteomics. 2014 Dec;11(6):697-711. doi: 10.1586/14789450.2014.971116. Epub 2014 Oct 18. Expert Rev Proteomics. 2014. PMID: 25327590 Free PMC article.
-
The role of cytomegalovirus in angiogenesis.Virus Res. 2011 May;157(2):204-11. doi: 10.1016/j.virusres.2010.09.011. Epub 2010 Oct 1. Virus Res. 2011. PMID: 20869406 Free PMC article. Review.
-
Cytokine-Mediated Induction and Regulation of Tissue Damage During Cytomegalovirus Infection.Front Immunol. 2019 Jan 29;10:78. doi: 10.3389/fimmu.2019.00078. eCollection 2019. Front Immunol. 2019. PMID: 30761144 Free PMC article. Review.
References
-
- Billingham, M. E. 1992. Histopathology of graft coronary disease. J. Heart Lung Transplant. 11S38-S44. - PubMed
-
- Bouis, D., Y. Kusumanto, C. Meijer, N. H. Mulder, and G. A. Hospers. 2006. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol. Res. 5389-103. - PubMed
-
- Bruning, J. H., M. C. J. Persoons, K. B. Lemstrom, F. S. Stals, E. De Clereq, and C. A. Bruggeman. 1994. Enhancement of transplantation associated atherosclerosis by CMV, which can be prevented by antiviral therapy in the form of HPMPC. Transplant. Int. 7365-370. - PubMed
-
- Carmeliet, P. 2003. Angiogenesis in health and disease. Nat. Med. 9653-660. - PubMed
-
- Charrier, L., Y. Yan, A. Driss, C. L. Laboisse, S. V. Sitaraman, and D. Merlin. 2005. ADAM-15 inhibits wound healing in human intestinal epithelial cell monolayers. Am. J. Physiol. Gastrointest. Liver Physiol. 288G346-G353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL71695/HL/NHLBI NIH HHS/United States
- R01 HL071695/HL/NHLBI NIH HHS/United States
- AI21640/AI/NIAID NIH HHS/United States
- R01 HL066238/HL/NHLBI NIH HHS/United States
- K01 RR000163/RR/NCRR NIH HHS/United States
- R37 AI021640/AI/NIAID NIH HHS/United States
- R01 AI021640/AI/NIAID NIH HHS/United States
- P51 RR000163/RR/NCRR NIH HHS/United States
- R01 HL085451/HL/NHLBI NIH HHS/United States
- HL083194/HL/NHLBI NIH HHS/United States
- RR00163/RR/NCRR NIH HHS/United States
- R01 HL083194/HL/NHLBI NIH HHS/United States
- R01 HL088603/HL/NHLBI NIH HHS/United States
- HL65754/HL/NHLBI NIH HHS/United States
- HL 66238-01/HL/NHLBI NIH HHS/United States
- R01 HL065754/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

