Cholesterol effectively blocks entry of flavivirus
- PMID: 18448543
- PMCID: PMC2447114
- DOI: 10.1128/JVI.00117-08
Cholesterol effectively blocks entry of flavivirus
Erratum in
-
Correction for Lee et al., "Cholesterol Effectively Blocks Entry of Flavivirus".J Virol. 2024 Oct 22;98(10):e0113124. doi: 10.1128/jvi.01131-24. Epub 2024 Aug 30. J Virol. 2024. PMID: 39212452 Free PMC article. No abstract available.
Abstract
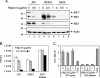
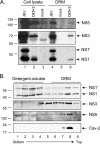
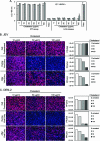
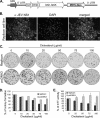
Japanese encephalitis virus (JEV) and dengue virus serotype 2 (DEN-2) are enveloped flaviviruses that enter cells through receptor-mediated endocytosis and low pH-triggered membrane fusion and then replicate in intracellular membrane structures. Lipid rafts, cholesterol-enriched lipid-ordered membrane domains, are platforms for a variety of cellular functions. In this study, we found that disruption of lipid raft formation by cholesterol depletion with methyl-beta-cyclodextrin or cholesterol chelation with filipin III reduces JEV and DEN-2 infection, mainly at the intracellular replication steps and, to a lesser extent, at viral entry. Using a membrane flotation assay, we found that several flaviviral nonstructural proteins are associated with detergent-resistant membrane structures, indicating that the replication complex of JEV and DEN-2 localizes to the membranes that possess the lipid raft property. Interestingly, we also found that addition of cholesterol readily blocks flaviviral infection, a result that contrasts with previous reports of other viruses, such as Sindbis virus, whose infectivity is enhanced by cholesterol. Cholesterol mainly affected the early step of the flavivirus life cycle, because the presence of cholesterol during viral adsorption greatly blocked JEV and DEN-2 infectivity. Flavirial entry, probably at fusion and RNA uncoating steps, was hindered by cholesterol. Our results thus suggest a stringent requirement for membrane components, especially with respect to the amount of cholesterol, in various steps of the flavivirus life cycle.
Figures





References
-
- Aizaki, H., K. J. Lee, V. M. Sung, H. Ishiko, and M. M. Lai. 2004. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology 324450-461. - PubMed
-
- Briggs, J. A., T. Wilk, and S. D. Fuller. 2003. Do lipid rafts mediate virus assembly and pseudotyping? J. Gen. Virol. 84757-768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

