Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration
- PMID: 18448544
- PMCID: PMC2447094
- DOI: 10.1128/JVI.00068-08
Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration
Abstract
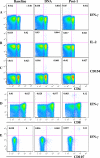
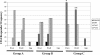
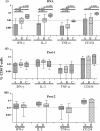
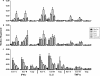
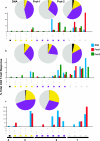
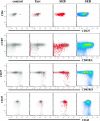
A phase I clinical vaccine study of a human immunodeficiency virus type 1 (HIV-1) vaccine regimen comprising a DNA prime formulation (5-valent env and monovalent gag) followed by a 5-valent Env protein boost for seronegative adults was previously shown to induce HIV-1-specific T cells and anti-Env antibodies capable of neutralizing cross-clade viral isolates. In light of these initial findings, we sought to more fully characterize the HIV-1-specific T cells by using polychromatic flow cytometry. Three groups of participants were vaccinated three times with 1.2 mg of DNA administered intradermally (i.d.; group A), 1.2 mg of DNA administered intramuscularly (i.m.; group B), or 7.2 mg of DNA administered i.m. (high-dose group C) each time. Each group subsequently received one or two doses of 0.375 mg each of the gp120 protein boost vaccine (i.m.). Env-specific CD4 T-cell responses were seen in the majority of participants; however, the kinetics of responses differed depending on the route of DNA administration. The high i.m. dose induced the responses of the greatest magnitude after the DNA vaccinations, while the i.d. group exhibited the responses of the least magnitude. Nevertheless, after the second protein boost, the magnitude of CD4 T-cell responses in the i.d. group was indistinguishable from those in the other two groups. After the DNA vaccinations and the first protein boost, a greater number of polyfunctional Env-specific CD4 T cells (those with > or = 2 functions) were seen in the high-dose group than in the other groups. Gag-specific CD4 T cells and Env-specific CD8 T cells were seen only in the high-dose group. These findings demonstrate that the route and dose of DNA vaccines significantly impact the quality of immune responses, yielding important information for future vaccine design.
Figures
References
-
- Ackers, M. L., B. Parekh, T. G. Evans, P. Berman, S. Phillips, M. Allen, and J. S. McDougal. 2003. Human immunodeficiency virus (HIV) seropositivity among uninfected HIV vaccine recipients. J. Infect. Dis. 187879-886. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. - PubMed
-
- Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8379-385. - PubMed
-
- Arbizu, E. A., R. B. Marugan, J. Y. Grijalba, P. L. Serrano, L. G. Grande, and S. Del Campo Terron. 2003. Intramuscular versus intradermal administration of anti-hepatitis B vaccine in non-cirrhotic hepatitis C patients. Vaccine 212747-2750. - PubMed
-
- Banatvala, J. E., and P. Van Damme. 2003. Hepatitis B vaccine—do we need boosters? J. Viral Hepat. 101-6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

